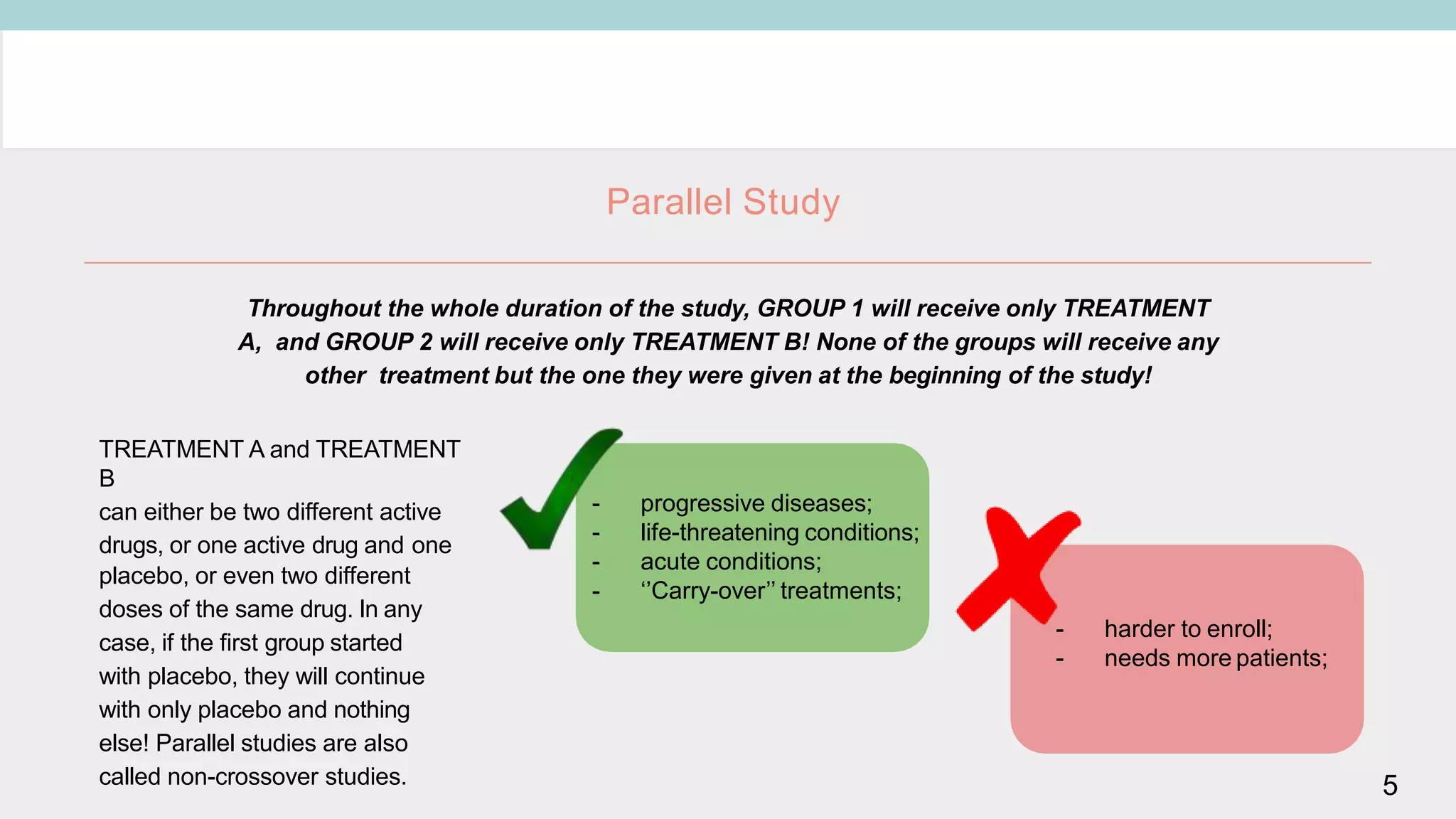
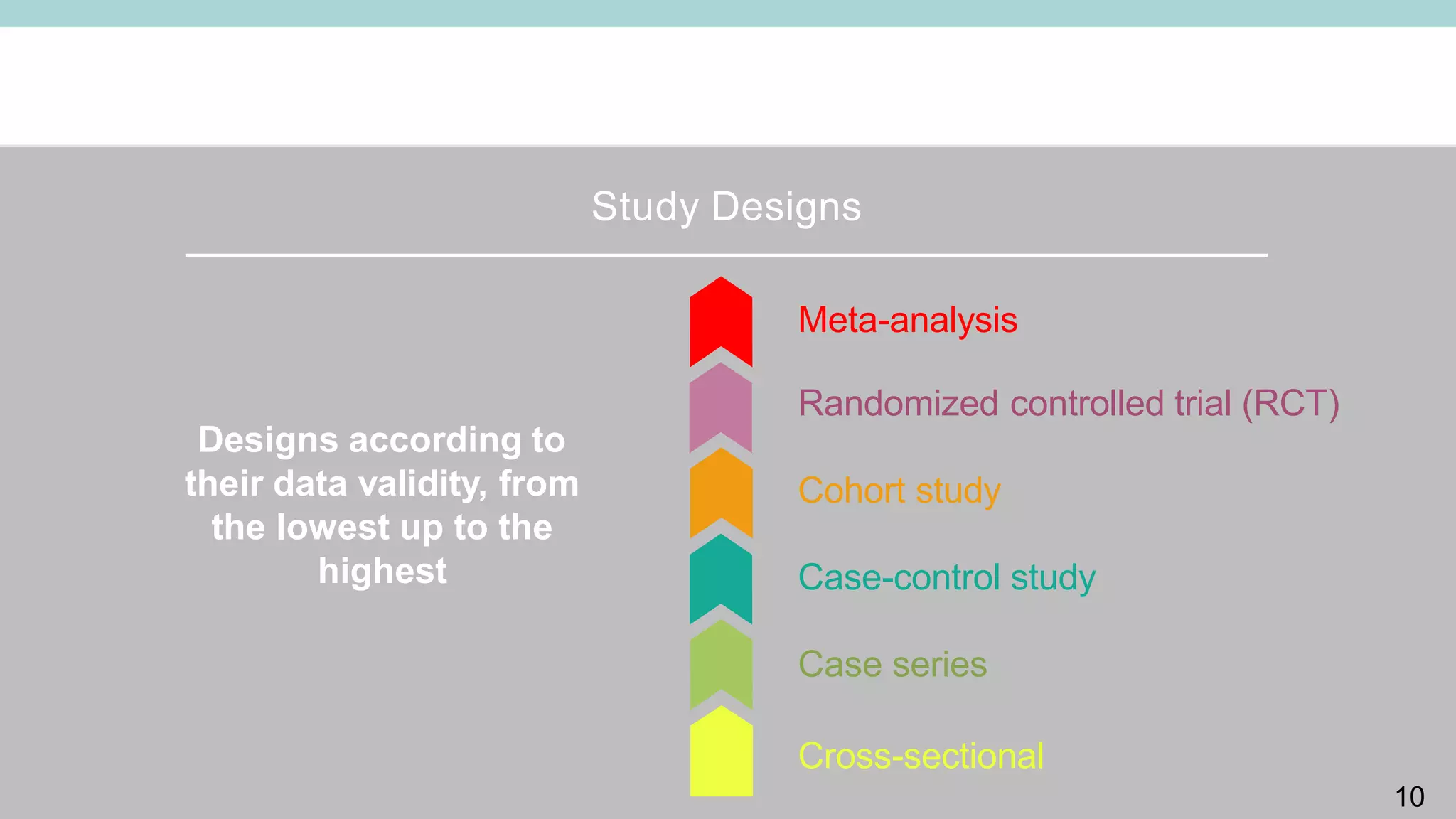
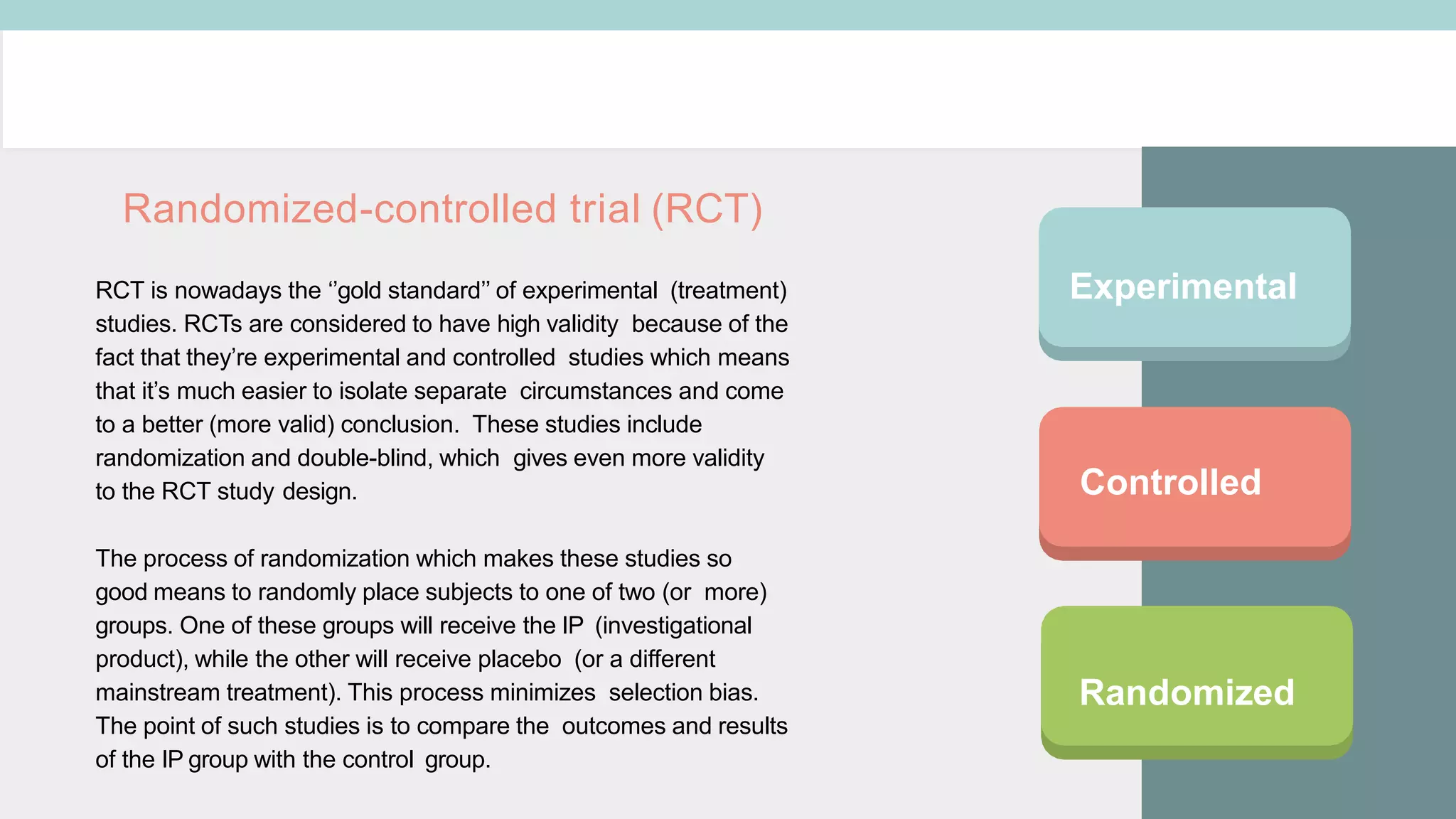
Clinical study designs can be categorized as either experimental (treatment) studies or observational studies. Experimental studies have more validity due to randomization and control groups. Common study designs include randomized controlled trials (RCTs), which are considered the gold standard; cohort studies, which follow groups over time; and case-control studies, which identify exposures in individuals with and without an outcome. Parallel and crossover studies differ in that parallel studies assign subjects to separate treatment groups throughout, while crossover studies switch subjects between treatments with washout periods between.