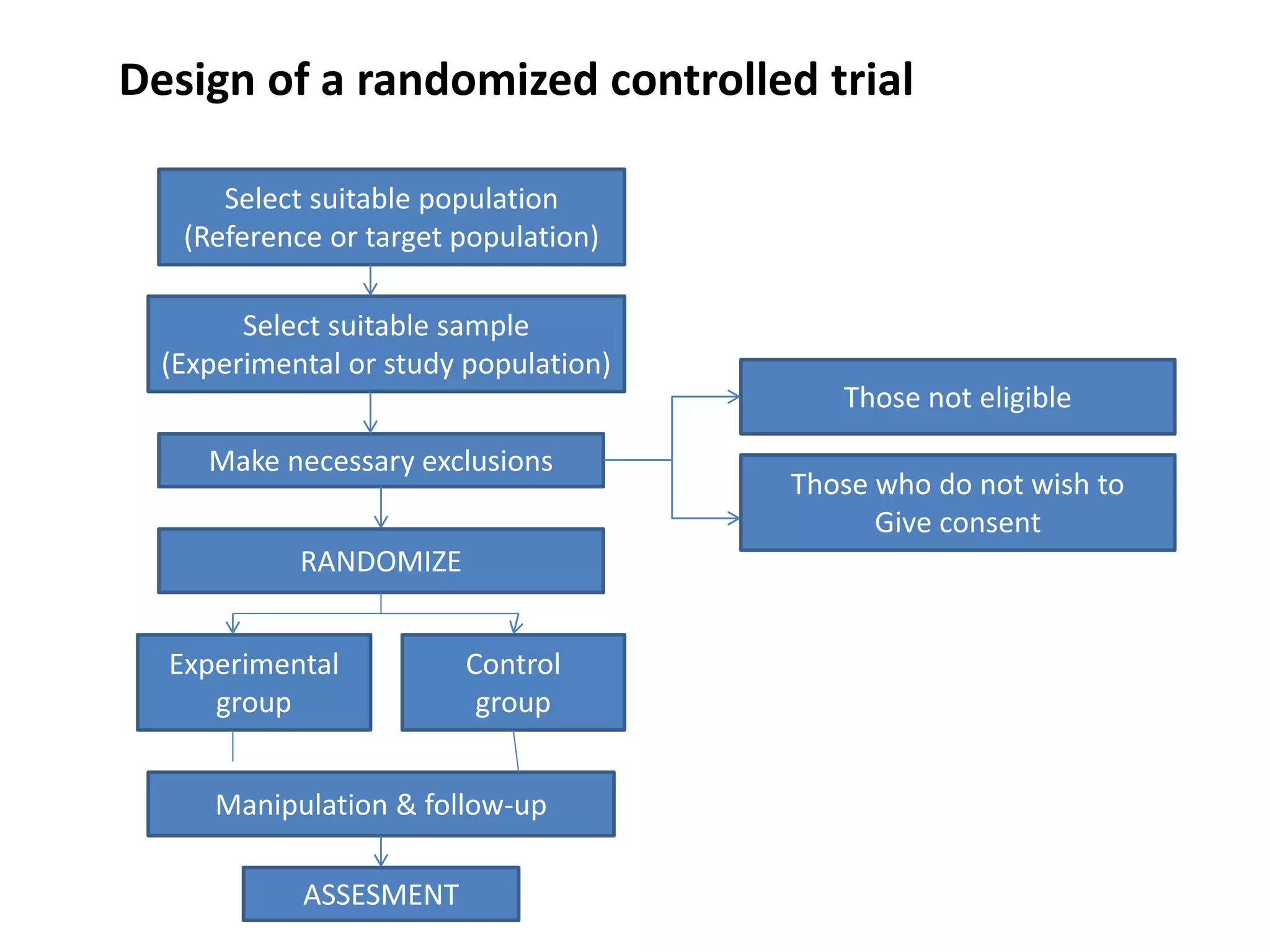
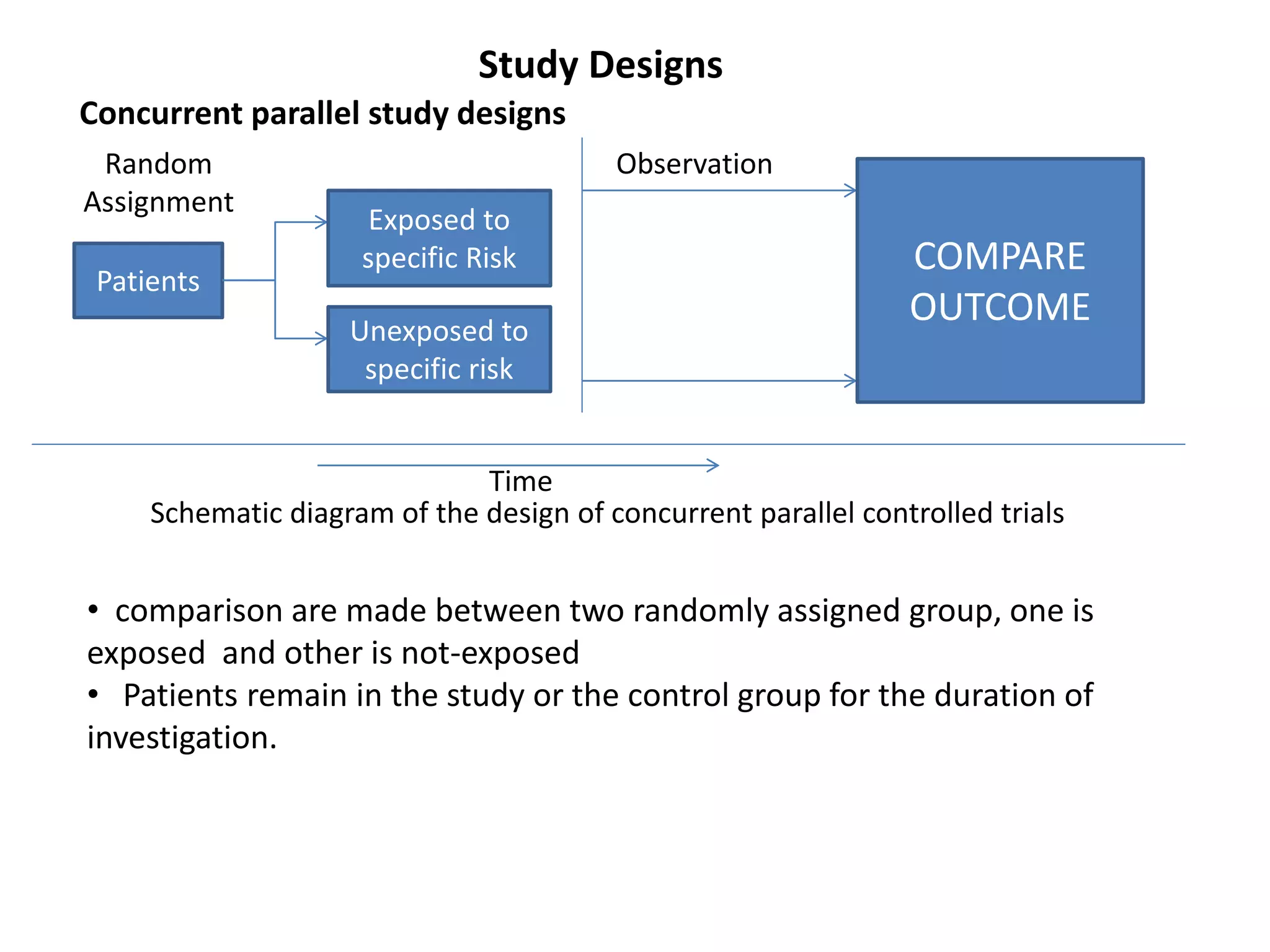
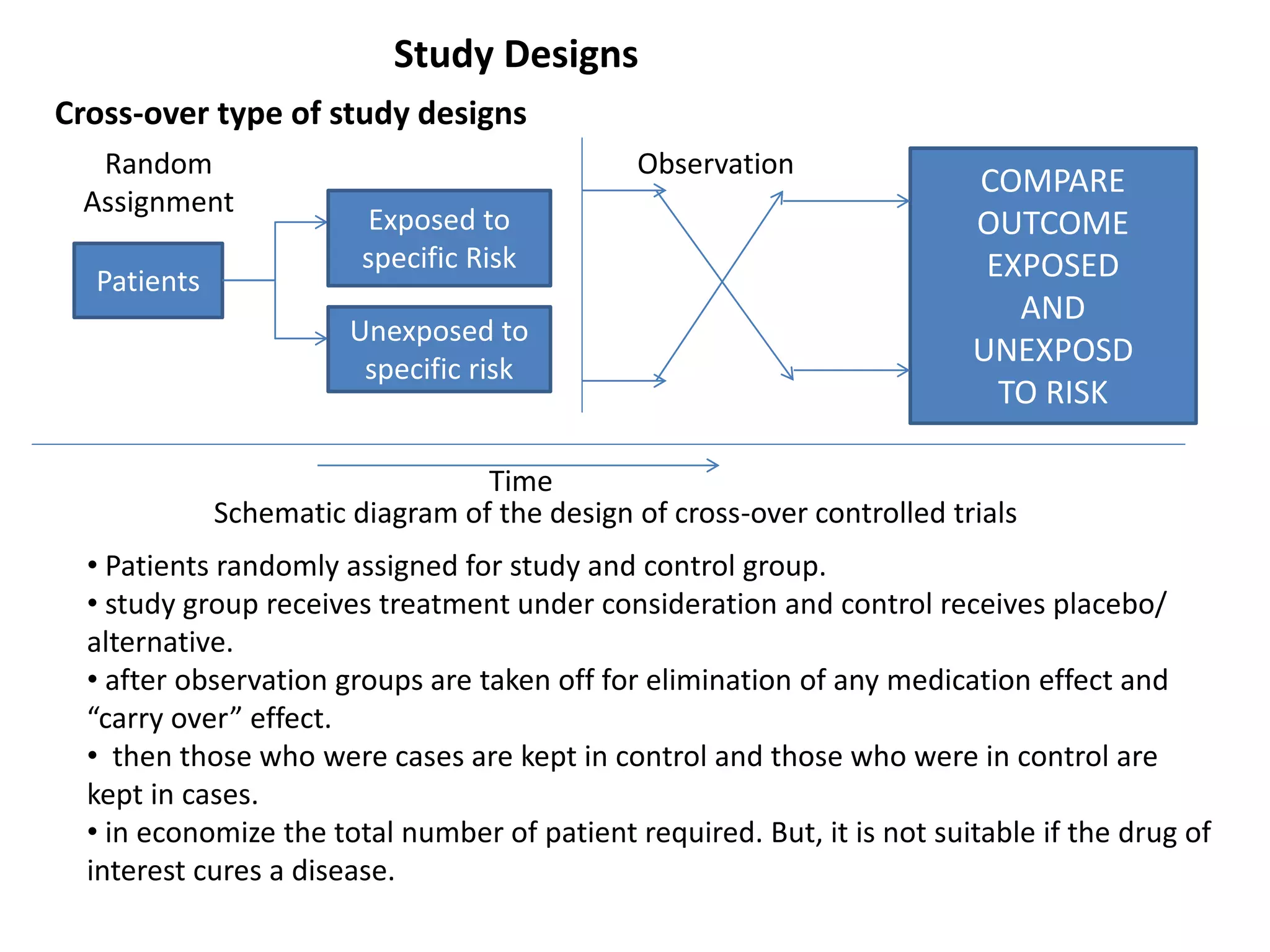
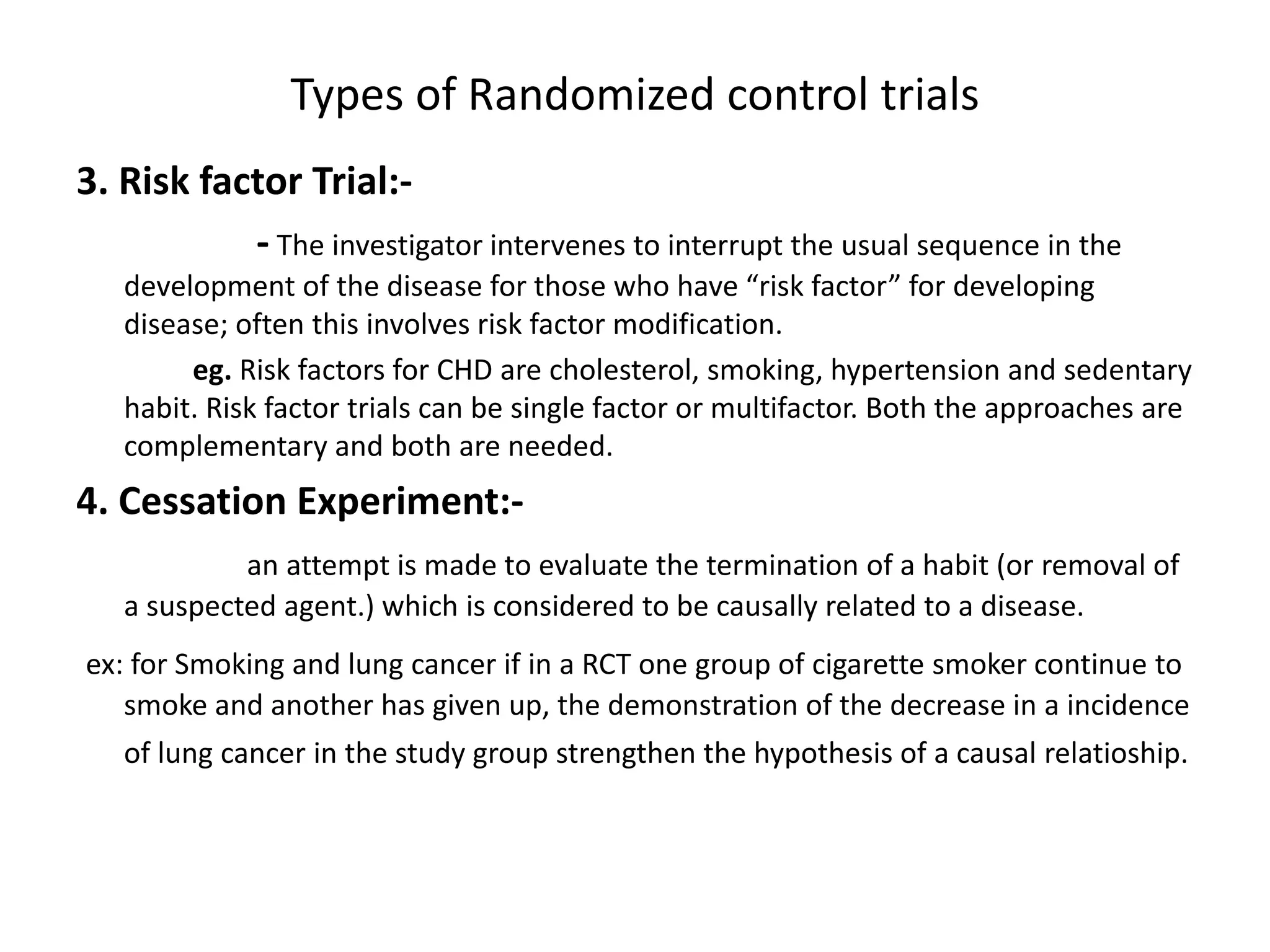
This study aims to prevent childhood obesity through early childhood feeding and parenting guidance. It will randomize pregnant women into an intervention and control group. The intervention group will receive home visits and guidance from community health workers, while the control group will only receive measurements. The study hypothesizes that fewer children in the intervention group will be overweight or obese due to differences in feeding practices and responsiveness to infant cues. Data on infant growth, diet, sleep and more will be collected to assess the effectiveness of the early intervention program in preventing childhood obesity.