This document outlines the process validation plan for a solid dosage anti-tuberculosis drug. It begins with an introduction and outline. It then discusses the stages of process validation, literature review, and plan of work. The document describes the manufacturing process and identifies critical and non-critical process parameters. It discusses sampling plans, statistical tools for analysis, and key references. The overall aim is to assure consistent quality and reduce defects through process validation.





![Validation conducted
prior to the
distribution of either
a new product or a
product made under a
revised
manufacturing
process
A process where
current production
batches are used to
monitor processing
parameter
Retrospective
validation is the
validation of a process
based on accumulated
historical data
PROSPECTIVE
VALIDATION
CONCURRENT
VALIDATION
RETROSPECTIVE
VALIDATION
TYPES OF PROCESS VALIDATION (1984 USFDA) [4]
7](https://image.slidesharecdn.com/fkjdshksdglfxk-161215091010/75/process-validation-7-2048.jpg)
![8
Continuous
process
verification
Continuous
Process
Verification
[1]](https://image.slidesharecdn.com/fkjdshksdglfxk-161215091010/75/process-validation-8-2048.jpg)
![INTENT TYPICAL ACTIVITIES
To define the commercial process
on knowledge gained through
development and scale up
activities .
The outcome is the design of a
process suitable for routine
manufacture that will consistently
deliver product that meets its
critical quality attributes
A combination of product and
process design (quality by design)
Product development activities
Experiment to determine process
parameters, variability, and
necessary controls
Risk assessments
Other activities required to define
the commercial process
Design of experiment testing
STAGE 1: PROCESS DESIGN [1]
9](https://image.slidesharecdn.com/fkjdshksdglfxk-161215091010/75/process-validation-9-2048.jpg)
![ To confirm the process design as
capable of reproducibility
Commercial manufacturing
Facilities design
Equipment and utilities
qualification
Performance qualification (PQ)
Strong emphasis on the use of
statistical analysis of process data
to understand process consistency
and performance.
INTENT TYPICAL ACTIVITIES
STAGE 2 : PROCESS QUALIFICATION [1]
10](https://image.slidesharecdn.com/fkjdshksdglfxk-161215091010/75/process-validation-10-2048.jpg)
![Installation
Qualification
Establish by objective evidence that all key
aspect of the process equipment and ancillary
system are adhere to manufacturing
specification
Operational
Qualification
Establish by objective evidence process control
limit and the action levels which result in
product that all predetermine requirement
Performance
Qualification
Establish by objective evidence that the process
,under anticipated conditions, consistently
produces product which meets all predetermine
requirement
STAGE 2: PROCESS QUALIFICATION [4]
11](https://image.slidesharecdn.com/fkjdshksdglfxk-161215091010/75/process-validation-11-2048.jpg)
![ To provide ongoing assurance that
the process remains in the state of
control during routine production
through procedure
Continuous improvement
initiatives
Proceduralised data collection
from every batch
Data trending and statistical
analysis
Product review
Equipment and facilities
maintenance
Calibration
Improvement initiatives through
process experience
INTENT TYPICAL ACTIVITIES
STAGE 3:CONTINUED PROCESS VERIFICATION [1]
12](https://image.slidesharecdn.com/fkjdshksdglfxk-161215091010/75/process-validation-12-2048.jpg)
![STAGE 1
PROCESS DESIGN
STAGE 2
PROCESS QUALIFICATION
(PQ)
DESIGN OF
FACILITIES &
QUALIFICATION
OF EQUIPMENT
AND UTILITIES
PROCESS
PERFORMANCE
QUALIFICATION
(PQR)
EVALUATE/ CONFIRM
STAGE 3
CONTINUED PROCESS VERIFICATION
(CPV)DISTRIBUTE
Three model of process validation according to FDA guidance for
industry [5]
distribute
13](https://image.slidesharecdn.com/fkjdshksdglfxk-161215091010/75/process-validation-13-2048.jpg)

![15
PARAMETER DESCRIPTION
IUPAC NAME
(7S,9E,11S,12R,13S,14R,15R,16R,17S,18S,19E,21Z)-
2,15,17,27,29-pentahydroxy-11-methoxy-3,7,12,14,16,18,22-
heptamethyl-26-[(1E)-[(4-methylpiperazin-1-yl)imino]methyl]-
6,23-dioxo-8,30-dioxa-24-
azatetracyclo[23.3.1.1⁴,⁷.0⁵,²⁸]triaconta-1(29),2,4,9,19,21,25,27-
octaen-13-yl acetate
MOLECULAR WEIGHT 822.9402
MOLECULAR FORMULA C43H58N4O12
MECHANISM OF ACTION
Rifampin acts via the inhibition of DNA-dependent RNA
polymerase, leading to a suppression of RNA synthesis and cell
death.
RIFAMPICIN :-](https://image.slidesharecdn.com/fkjdshksdglfxk-161215091010/75/process-validation-15-2048.jpg)

![17
PARAMETER DESCRIPTION
IUPAC NAME
(2S)-2-[(2-{[(2S)-1-hydroxybutan-2-
yl]amino}ethyl)amino]butan-1-ol
MOLECULAR WEIGHT 204.3098
MOLECULAR FORMULA C10H24N2O2
MECHANISM OF ACTION
Ethambutol inhibits arabinosyl transferases which is involved
in cell wall biosynthesis. By inhibiting this enzyme, the
bacterial cell wall complex production is inhibited. This leads
to an increase in cell wall permeability.
ETHAMBUTOL :-](https://image.slidesharecdn.com/fkjdshksdglfxk-161215091010/75/process-validation-17-2048.jpg)





![VALIDATION PROTOCOL [5]
Process validation protocols should include the following elements:
• Requirements for calibration of all measuring devices.
24
• Objectives, scope of coverage of the validation study.
• A list of all equipment to be used; their normal and worst case operating
parameters.](https://image.slidesharecdn.com/fkjdshksdglfxk-161215091010/75/process-validation-24-2048.jpg)

![Raw material dispensing
Sifting
Dry mixing
Binder preparation
Wet granulation
Drying
Sizing
Blend uniformity
% LOD
GENERAL MANUFAGTURING PROCESS OF TABLET [6,7,8]
26](https://image.slidesharecdn.com/fkjdshksdglfxk-161215091010/75/process-validation-26-2048.jpg)

![Manufacturing
Process Stage
Process Parameter
Critical/
Non-
critical
Justification
Sifting Sieve used for sifting (mesh size)
Non-
critical
Sifting has been
incorporate to break the
lump to remove foreign
particle if any.
Dry mixing
1. Dry mixing time
2. Impeller speed
Critical
It may have impact on
uniform distribution of
active material and
excipient
Wet granulation
Binder
addition
1. Add time
2. Impeller speed
Critical
Have impact on
homogeneity of
granulation process,
dissolution and physical
properties of granules
such as particle size
distribution and
followability
Kneading
1. Mix time
2. Impeller speed
3. Chopper sped
CRITICALAND NON-CRITICAL PARAMETER [6]
28](https://image.slidesharecdn.com/fkjdshksdglfxk-161215091010/75/process-validation-28-2048.jpg)


![• A detailed plan of sampling procedure of samples which shall be
analyzed/monitored during validation run shall be outline systematically
• The sampling plan including sampling points number of sample and the
frequency of sampling for each stage operation shall be decided based on
characteristic of the product or critical point of equipment
• Sampling location are to clearly indicate by diagram for any equipment from
which the sample are withdraw.
SAMPLING [7,8,10]
31](https://image.slidesharecdn.com/fkjdshksdglfxk-161215091010/75/process-validation-31-2048.jpg)
![Rapid Mix Granulator
T- top M- middle B-bottom
FBD Bowl
Four corner 1,2,3,4
SAMPLING TECHNIQUE [7,8]
32](https://image.slidesharecdn.com/fkjdshksdglfxk-161215091010/75/process-validation-32-2048.jpg)
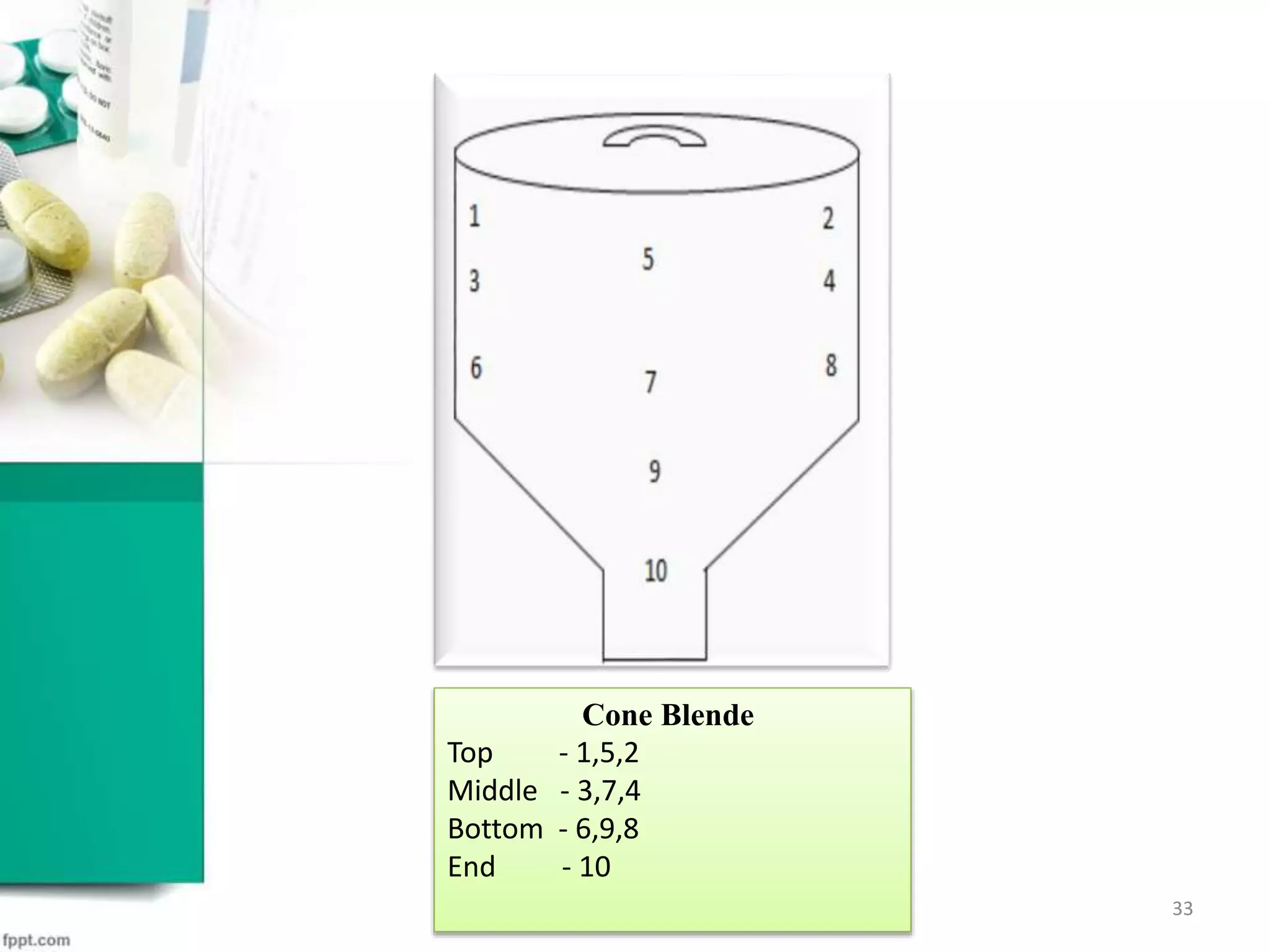
![Stage Sampling Location Test To Be Performed
Dry mixing
3 point from top,3 point from
middle, 3 point from bottom of
bed from RMG
Blend uniformity
Drying Four corner of FBD and middle % LOD
Lubrication and
Blending
3 samples from top, middle,
bottom
Assay for BU, tapped density
Bulk density, angle of repose
Compression
Draw tablets from initial middle ,
and near end stage of compression
Description, assay, hardness,
thickness, diameter,
friability, weight variation,
CU, disintegration,
dissolution
Coating
Draw a tablets from coating pan at
the end of each lot
Same as above and related
substance test
SAMPLING AND ANALYSIS [6,7]
34](https://image.slidesharecdn.com/fkjdshksdglfxk-161215091010/75/process-validation-34-2048.jpg)
![STATISTICS IN VALIDATION [8,9]
“When you can measure what you are speaking about, and express
it in numbers, then you know something about it.”[8]
Statistical process control (SPC), also called statistical quality
control and process validation (PV), represents two sides of the
same coin. [8] 35](https://image.slidesharecdn.com/fkjdshksdglfxk-161215091010/75/process-validation-35-2048.jpg)
![ The collection and evaluation of information and data about performance of
the process will allow detection of undesired process variability
Evaluating the performance of the process identifies problem and
determine weather action must be taken to correct anticipate and prevent
problem so that the process remain in control
PROCESS VARIABILITY [9]
36](https://image.slidesharecdn.com/fkjdshksdglfxk-161215091010/75/process-validation-36-2048.jpg)
![STATISTICAL TOOL IN VALIDATION
Process Capability [9,10]
37](https://image.slidesharecdn.com/fkjdshksdglfxk-161215091010/75/process-validation-37-2048.jpg)

![ Control charts are used to detect changes in the process.
Control charts help to identify key input variables causing the process to shift
and aid in the reduction of the variation. Control charts are also used as part of
a capability study to demonstrate that the process is stable or consistent.
Control Chart [9,10]
39](https://image.slidesharecdn.com/fkjdshksdglfxk-161215091010/75/process-validation-39-2048.jpg)


