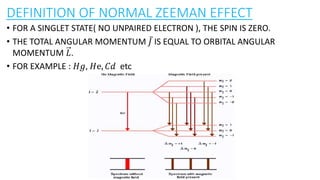
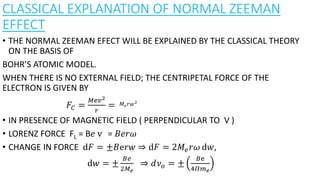
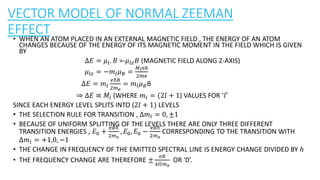
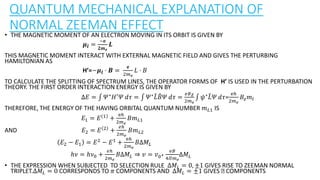
The document discusses the Zeeman effect, which is the splitting of a spectral line into multiple components when exposed to a magnetic field, first discovered by Pieter Zeeman in 1896. It outlines the types of Zeeman effects, namely the normal and anomalous effects, and provides both classical and quantum mechanical explanations for the normal Zeeman effect. The document also includes historical context, definitions, and mathematical descriptions related to the phenomenon.