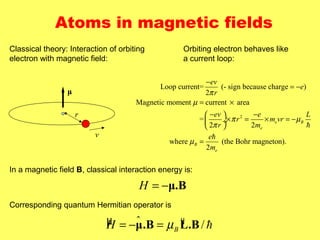
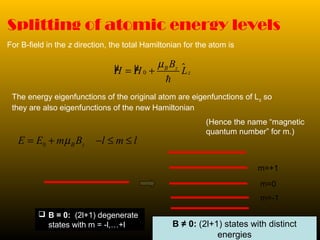
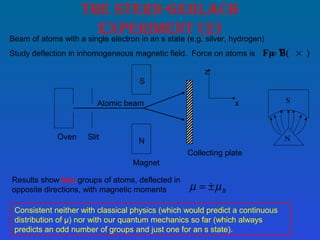
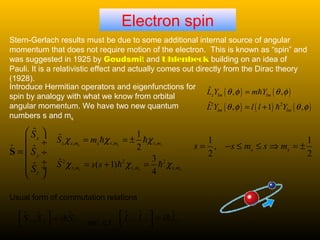
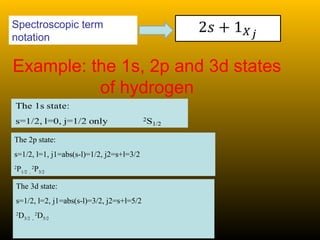
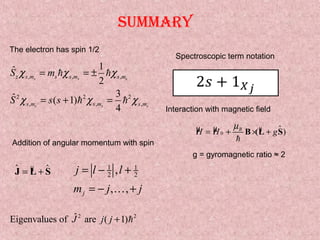
This document summarizes the key concepts of angular momentum of electrons and the Stern-Gerlach experiment. It discusses how electrons have both orbital angular momentum from their motion around the nucleus, as well as intrinsic spin angular momentum. The Stern-Gerlach experiment provided evidence of electron spin by observing the splitting of a silver atom beam in a non-homogeneous magnetic field. When combining multiple angular momenta, the total angular momentum and its quantum numbers are obtained by adding the angular momentum vectors or their corresponding quantum numbers. This allows predicting the energy levels and degeneracies of atomic orbitals.