This document discusses xanthan gum, a biopolymer produced through the fermentation of glucose by the bacterium Xanthomonas campestris. It provides information on:
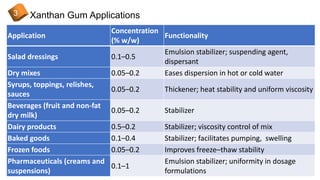
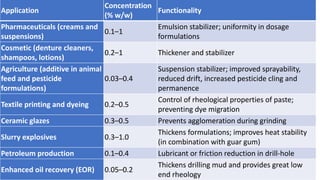
1) Xanthan gum's properties, structure, production process, and applications in foods, cosmetics, and industrial products like drilling fluids and textiles.
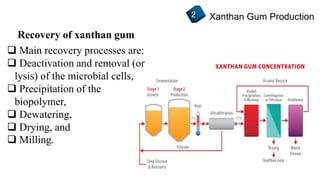
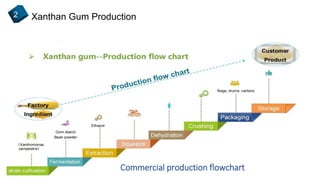
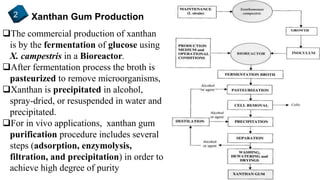
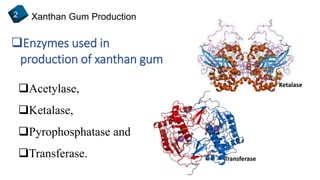
2) How xanthan gum is commercially produced through the fermentation of glucose in a bioreactor, followed by recovery processes to extract and purify the biopolymer.
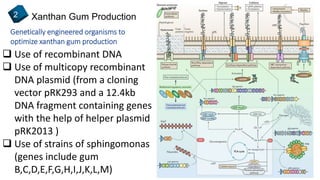
3) Ongoing research seeking to improve xanthan gum production through genetic engineering of bacteria strains and the use of alternative carbon sources for fermentation.



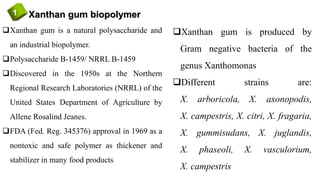
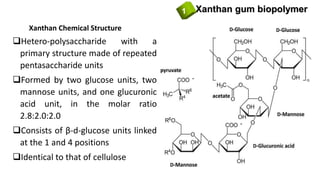
![Xanthan gum biopolymer1
Biopolymers structures
Biopolymers Overview
Chitosan (biopolymer)[1]
[2]](https://image.slidesharecdn.com/xanthangum1-200513010127/85/Xanthan-Gum-Biopolymer-4-320.jpg)
![Xanthan gum biopolymer1
Eco-bacterial polymer granules as biomaterials [2]](https://image.slidesharecdn.com/xanthangum1-200513010127/85/Xanthan-Gum-Biopolymer-5-320.jpg)
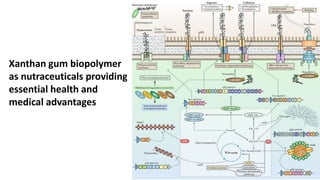
![Xanthan gum biopolymer1
Enhanced biopolymers
and biopolymer
synthesis as a target
for drug discovery [2]](https://image.slidesharecdn.com/xanthangum1-200513010127/85/Xanthan-Gum-Biopolymer-6-320.jpg)



![Xanthan Gum Production2
Xanthomonas cells
occur as single straight
rods, 0.4–0.7μm wide
and 0.7–1.8 μm long.
The cells are motile,
Gram-negative, and
they have a single polar
flagellum (1.7–3 μm
long)
Bacteria D-Glucose
D-
Mannose
D-Glucuronic
acid
Pyruvate Acetate
X. campestris 30.1 27.3 14.9 7.1 6.5
X. fragaria 1822 24.6 26.1 14.0 4.9 5.5
X.
gummisudans 21
82
34.8 30.7 16.5 4.7 10.0
X. juglandis 411 33.2 30.2 16.8 6.9 6.4
X. phaseoli 1128 30.9 28.6 15.3 1.8 6.4
X.
vasculorum 702
34.9 30.2 17.9 6.6 6.3
Microorganism
Mean %composition of polysaccharides by Xanthomonas
bacteria [3]](https://image.slidesharecdn.com/xanthangum1-200513010127/85/Xanthan-Gum-Biopolymer-13-320.jpg)
![Xanthan Gum Production2
Genetic engineered
Xanthan bacteria
pathway[4]](https://image.slidesharecdn.com/xanthangum1-200513010127/85/Xanthan-Gum-Biopolymer-15-320.jpg)
![Xanthan Gum Production2
Growth medium/nutrient[5]
S/NO Medium Ranged used (g.l-1) S/NO Medium Ranged used (g.l-1)
1 Glucose 10-42 13 CaCO3 0.020-20
2 Saccharose 1.125-50 14 CaCl2.2H2O 0.002
3 CH4N2O 0.10 15 Malt extract 3
4 Citric acid 2.0-2.1 16 Yeast extract 3-10
5 NH4NO3 0.217-1.144 17 Peptone 0.34
6 KH2PO4 1.0-5.0 18 Tryptone 10
7 MgSO4.7H2O 0.25-20 19 Soybean flour 15
8 NH4Cl 1.94 20 HCl 0.13-0.16
9 H3BO3 0.006 21 MgCl2 0.507
10 Na2HPO4 0.089 22 ZnO 0.006
11 ZnSO4 0.002 23 (NH4)2HPO4 0.217-1.5
12 FeCl3.6H2O 0.0042-0.024 24 MgSO4.7H2O 0.25-0.3](https://image.slidesharecdn.com/xanthangum1-200513010127/85/Xanthan-Gum-Biopolymer-17-320.jpg)