The document outlines a presentation on wheat and protein, including:
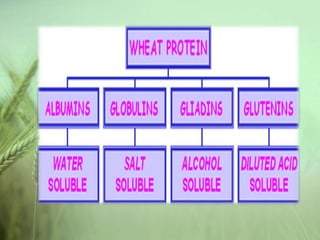
- Defining wheat and its four basic proteins: gliadin, glutenin, and deamidated gluten
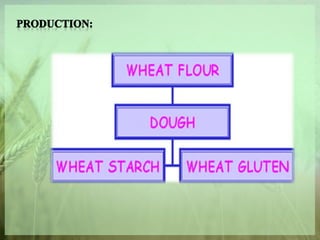
- The production of wheat starch and functional properties of wheat protein
- Details on the specific types and roles of gliadin and glutenin in forming gluten and giving bread its structure during baking.