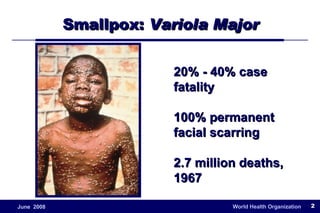
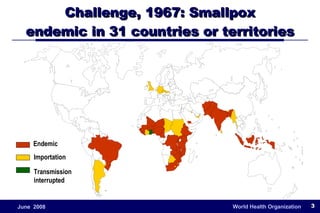
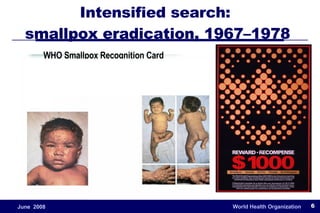
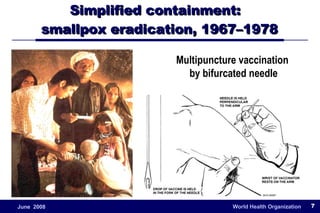
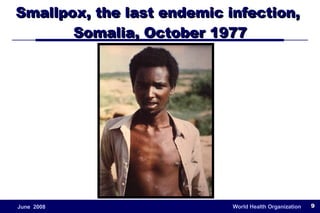
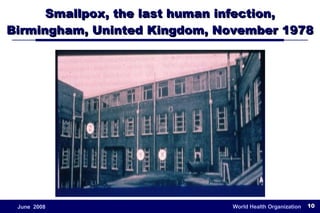
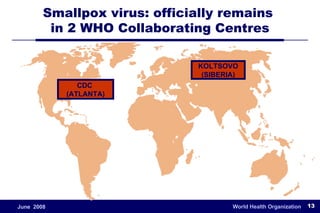
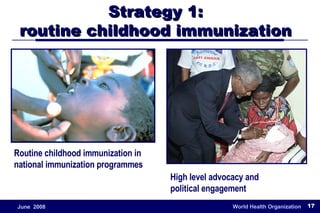
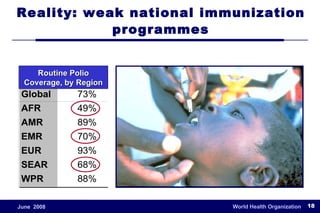
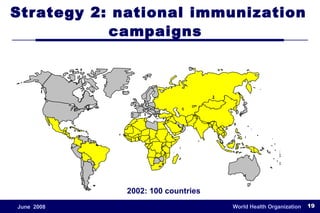
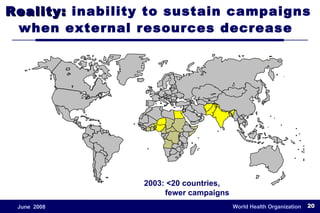
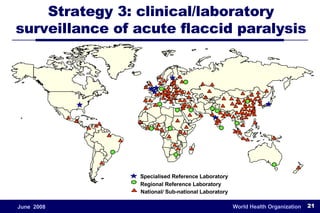
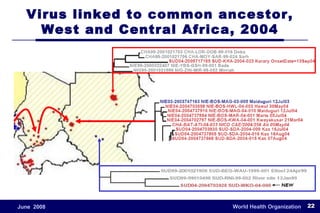
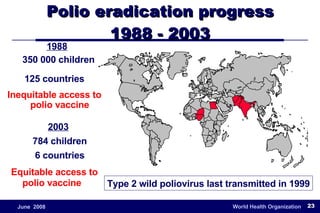
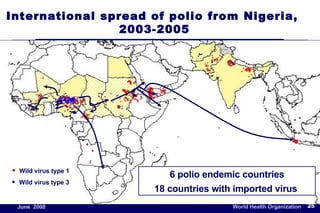
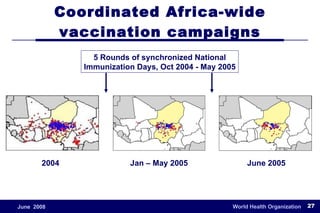
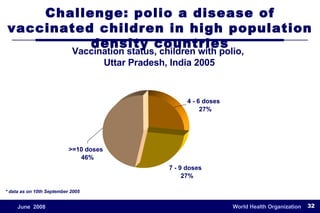
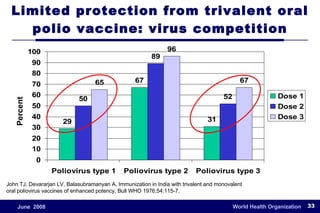
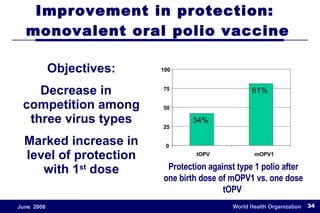
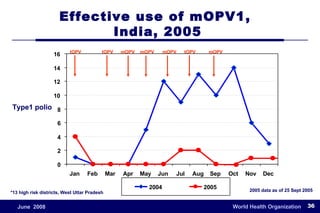
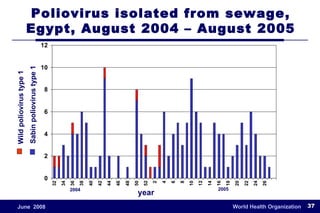
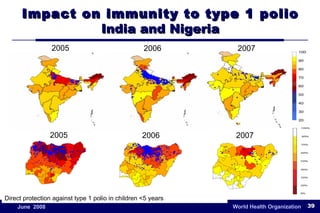
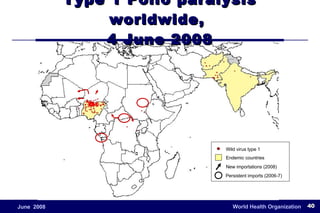
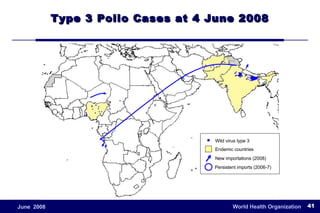
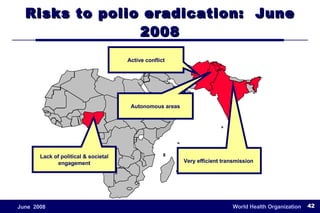
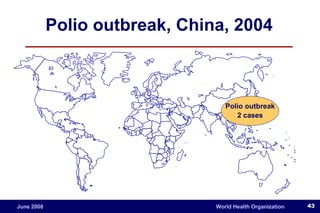
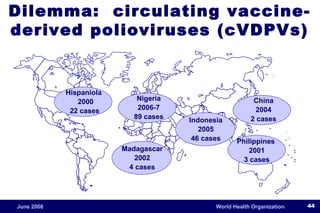
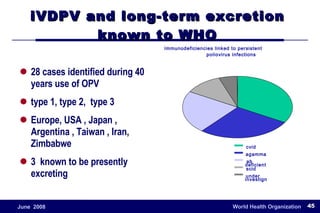
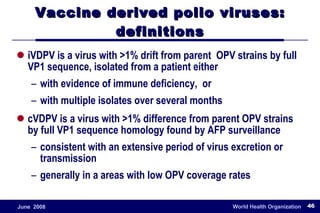
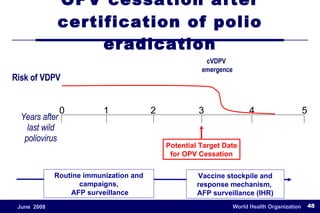
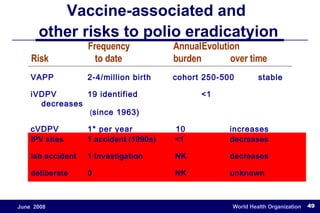
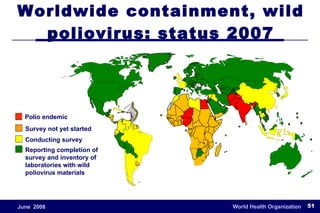
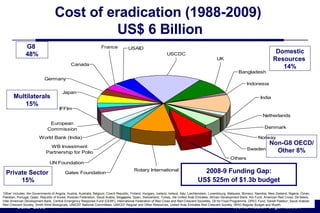
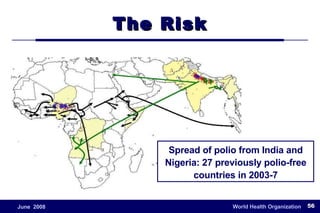
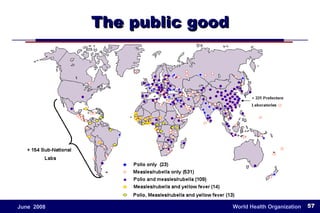
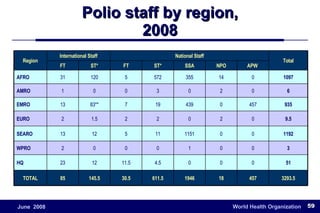
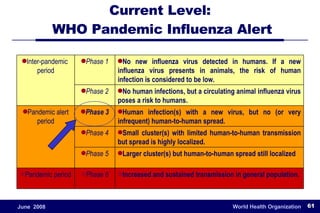
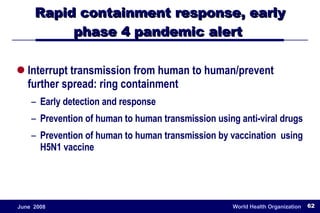
The document outlines the historical efforts and challenges in eradicating polio, summarizing strategies implemented from 1988 to 2008, including vaccination campaigns and political advocacy. It also highlights the successful eradication of smallpox by 1980, emphasizing the importance of global partnerships and vaccination in disease containment. With ongoing risks from vaccine-derived polioviruses and challenges in immunization sustainability, the document stresses the necessity for increased political commitment and resources to achieve complete polio eradication.