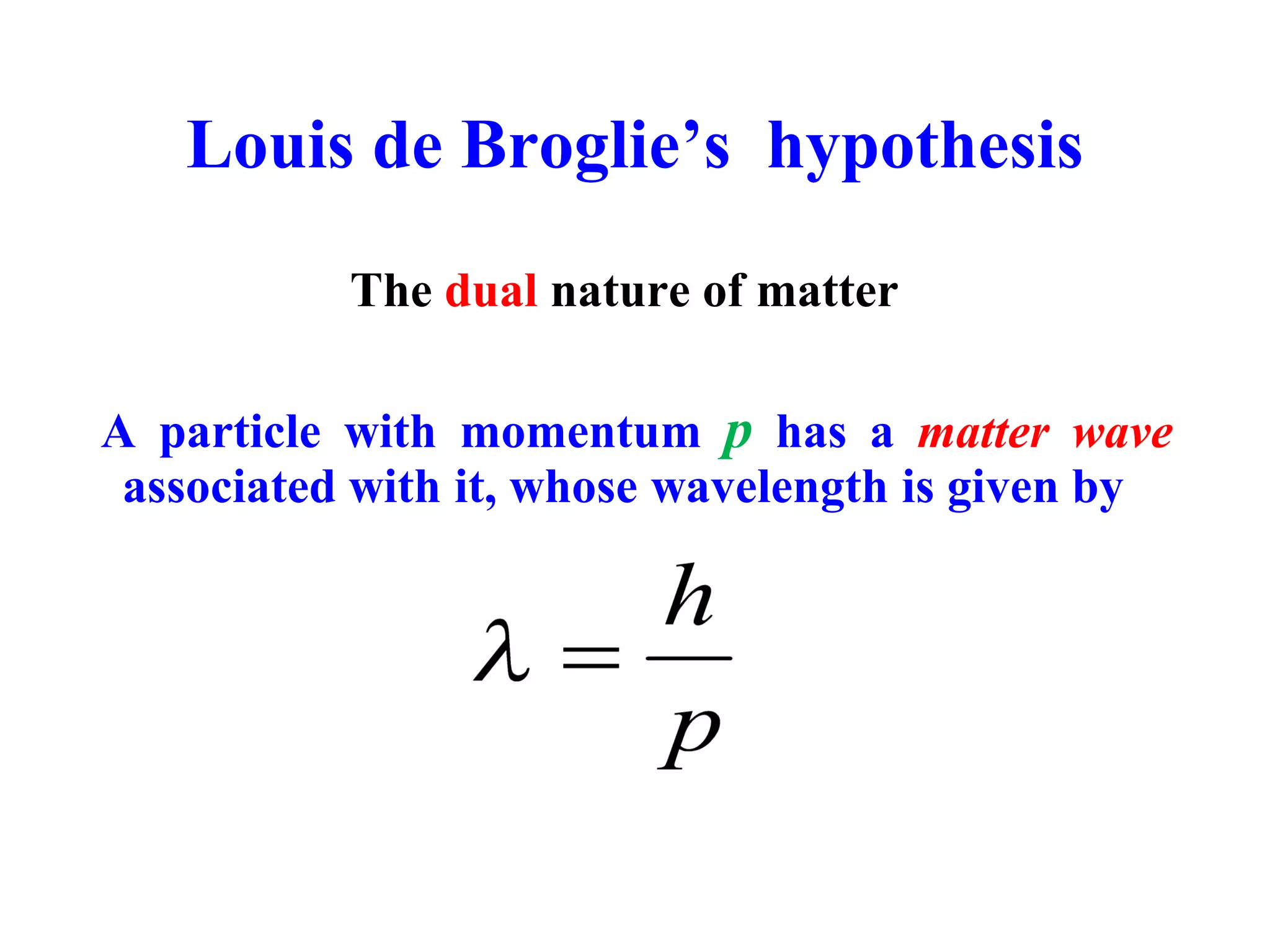
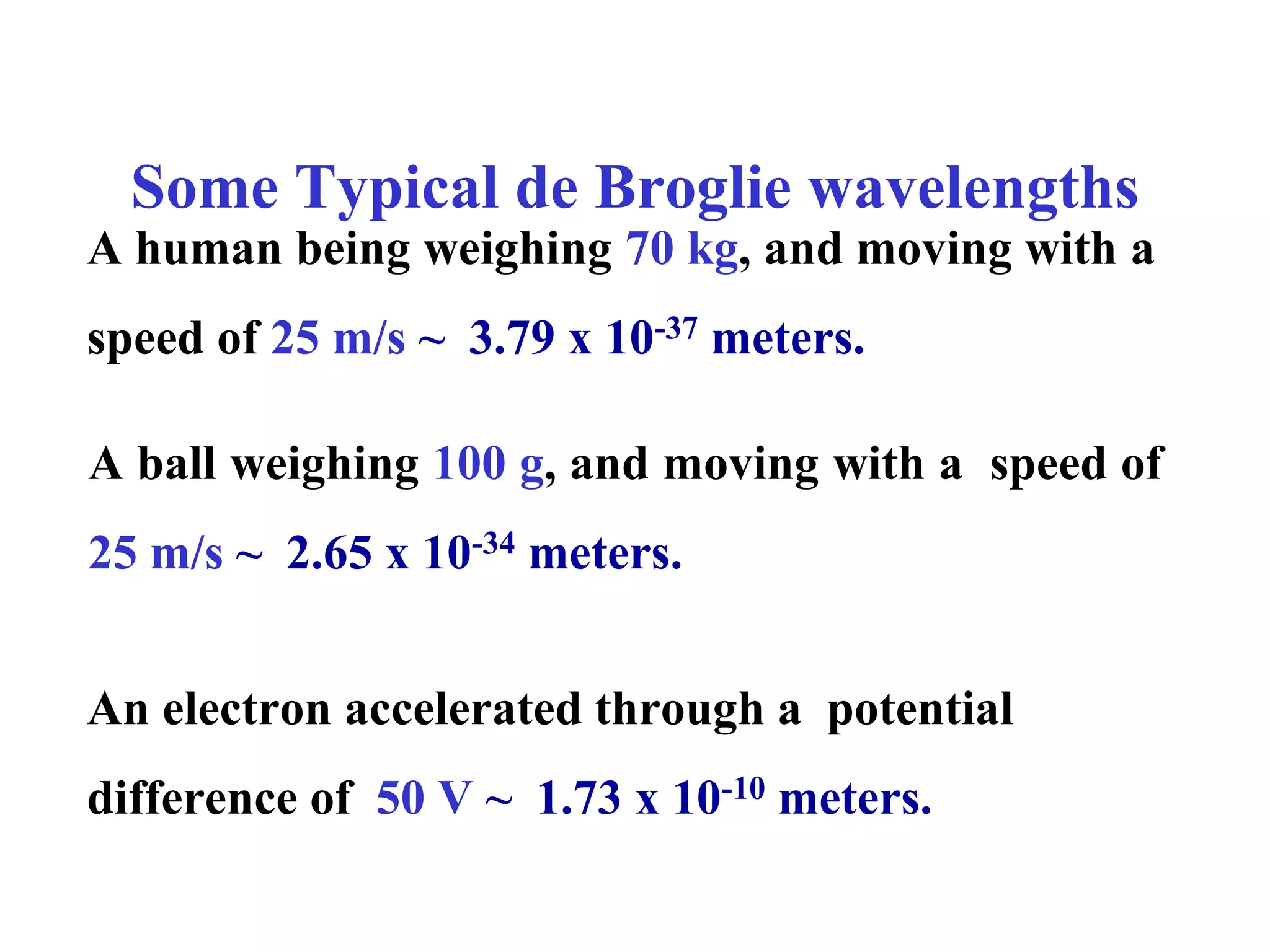
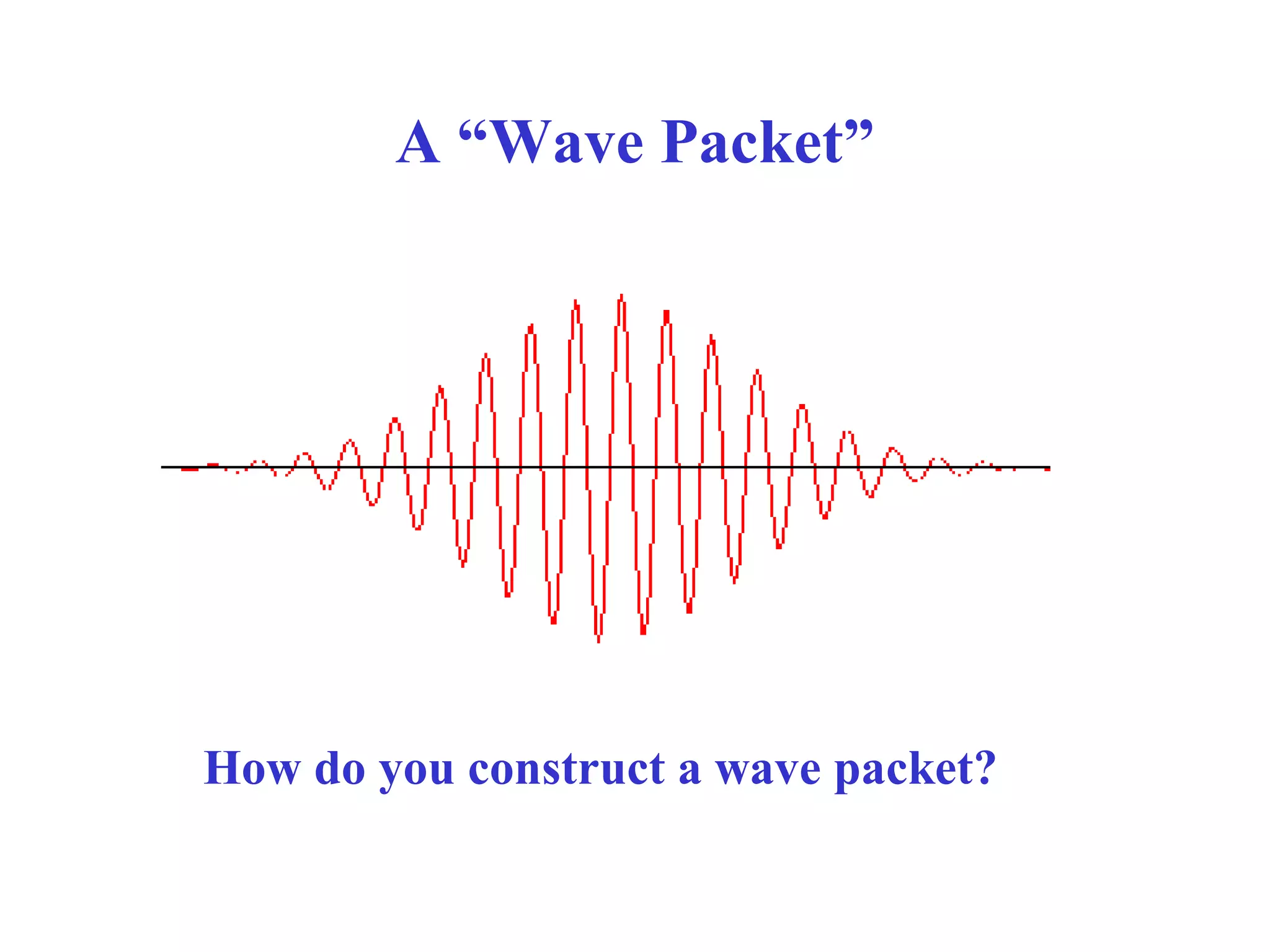
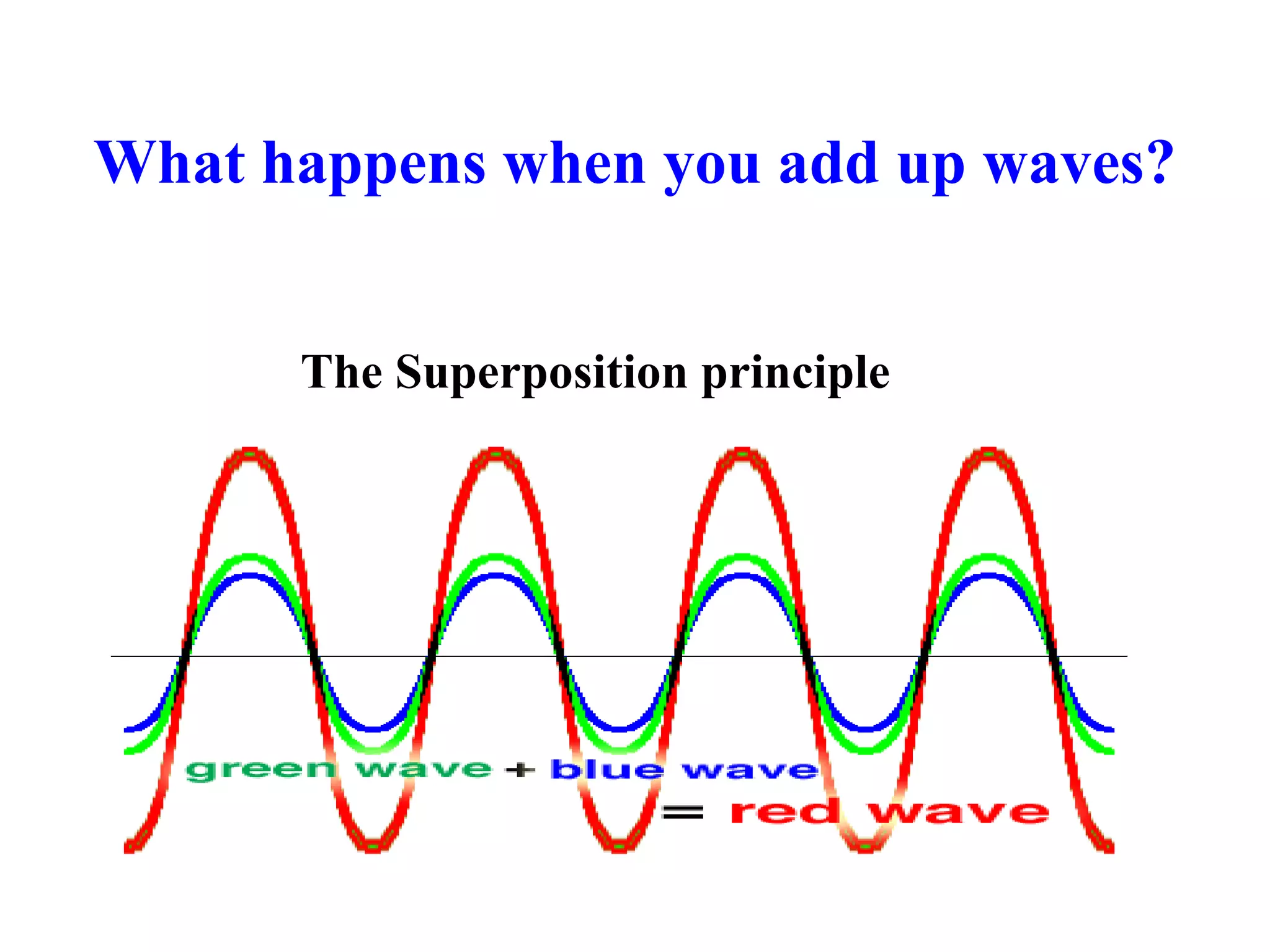
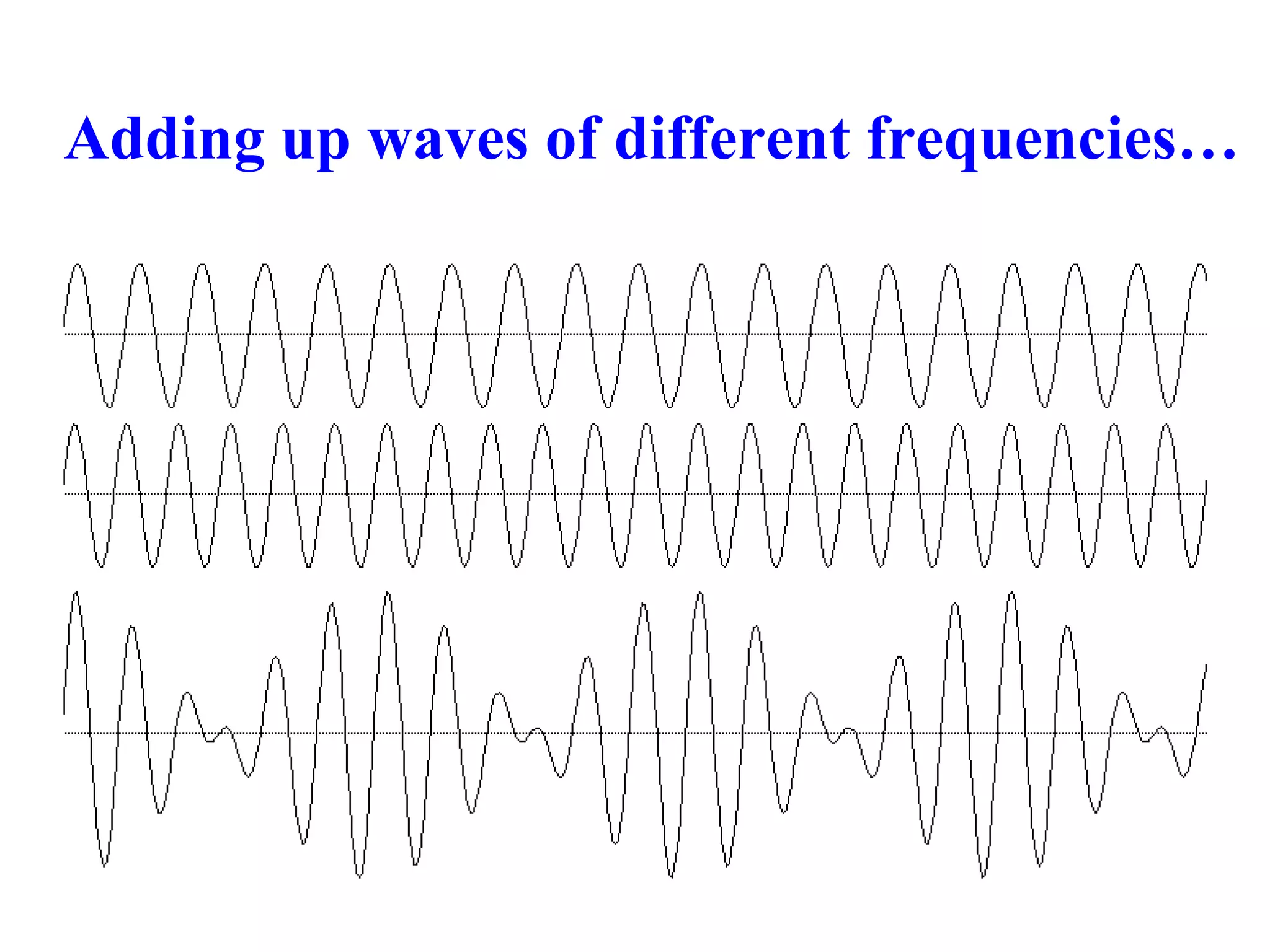
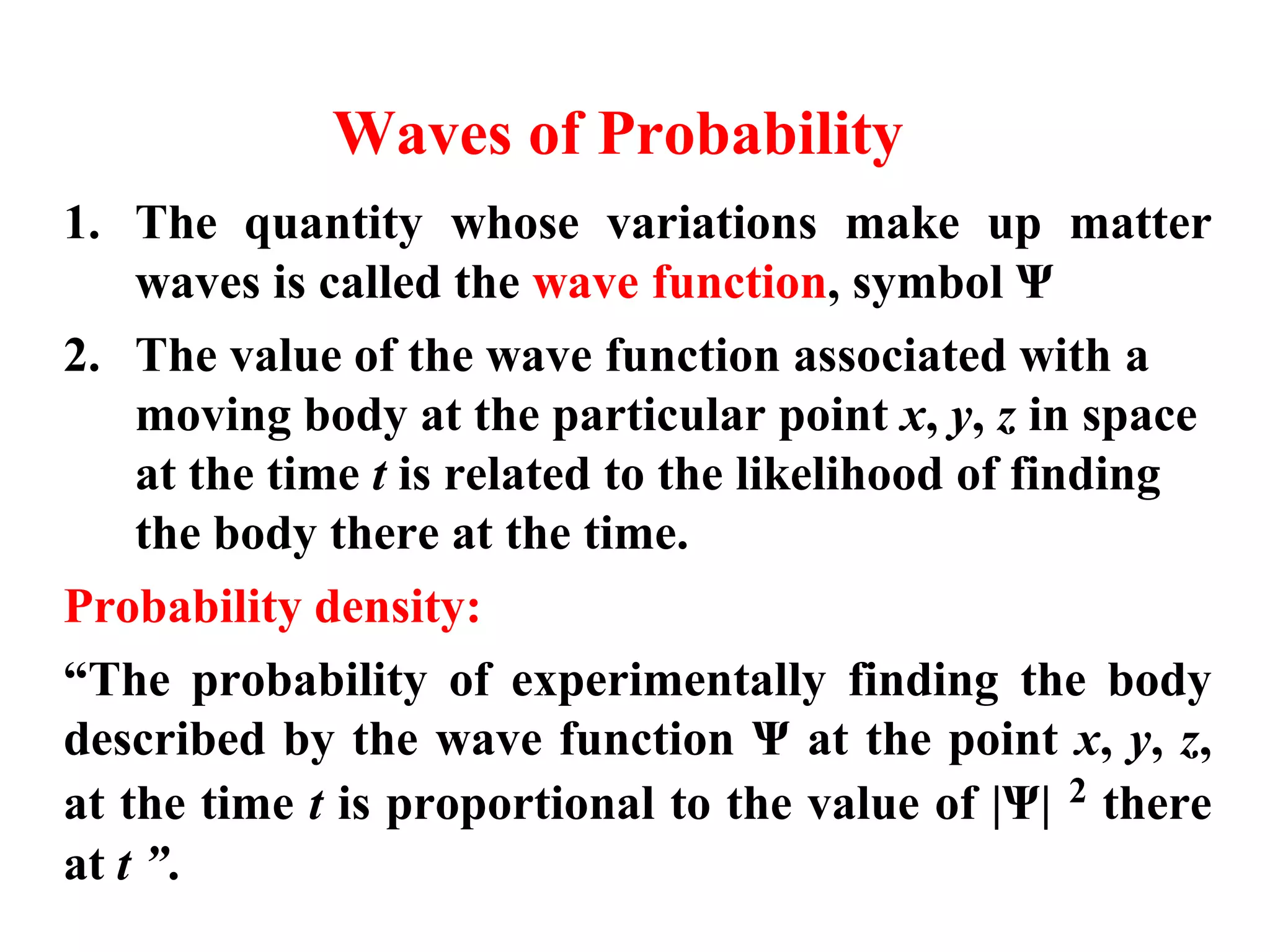
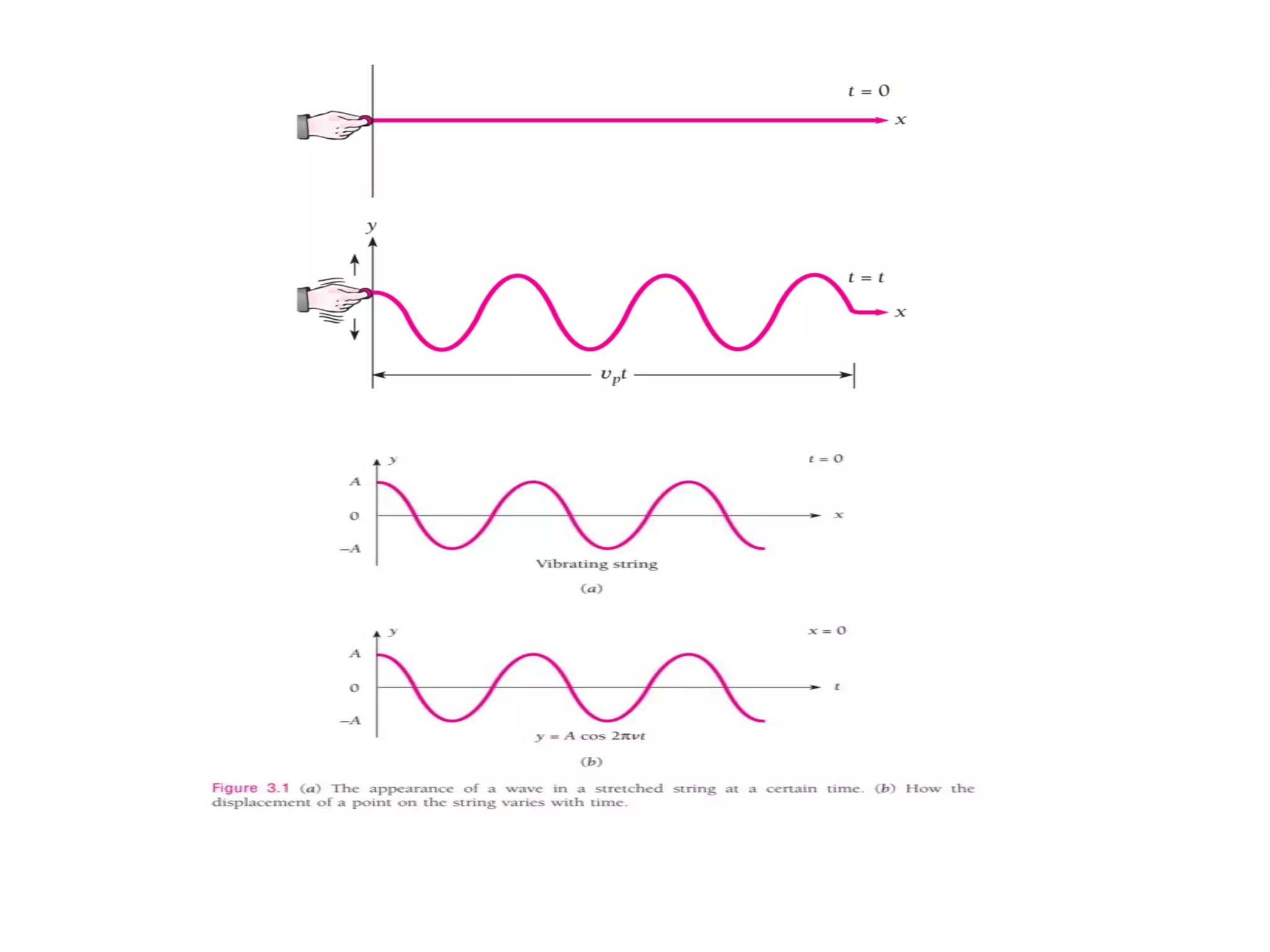
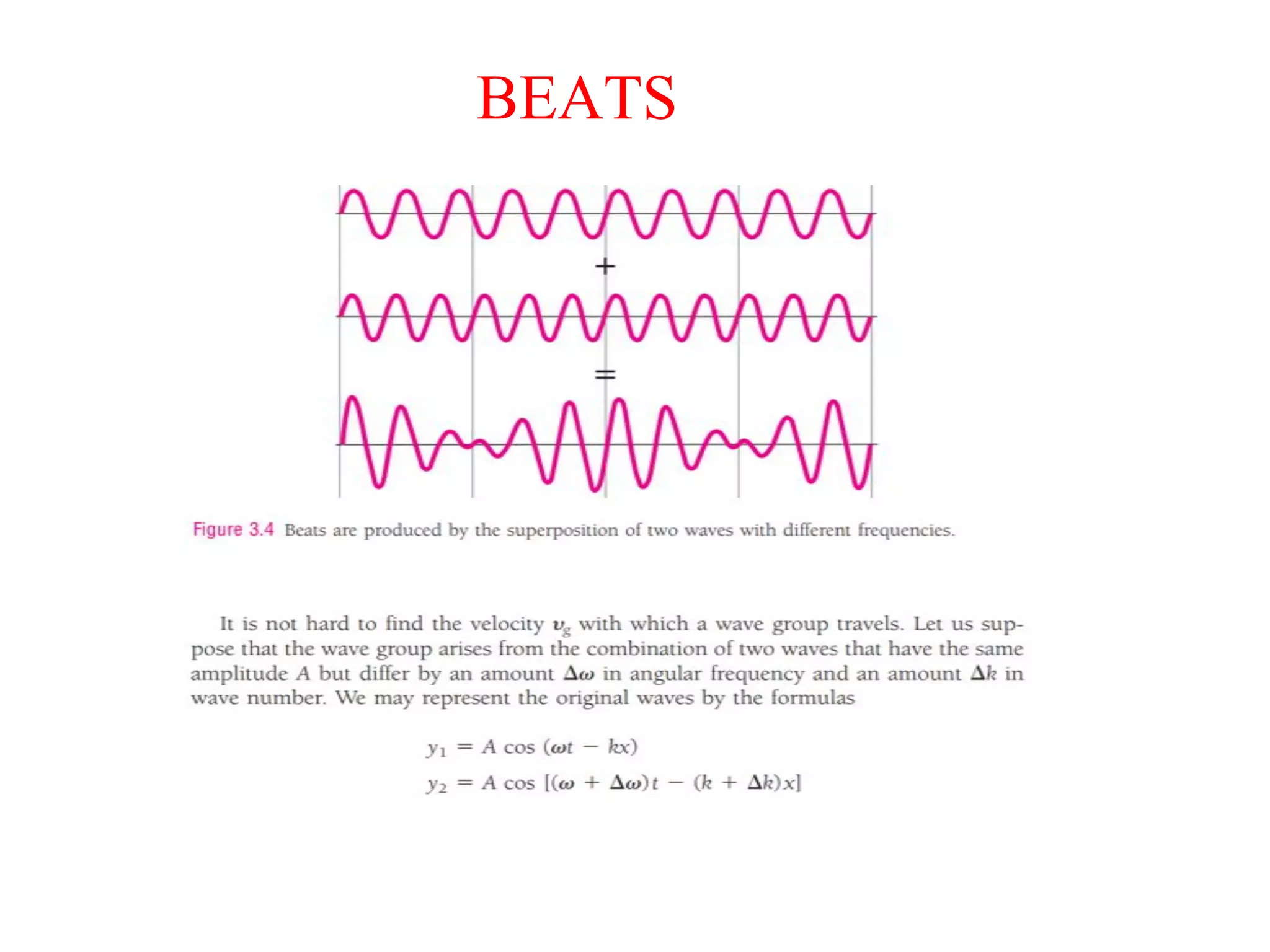
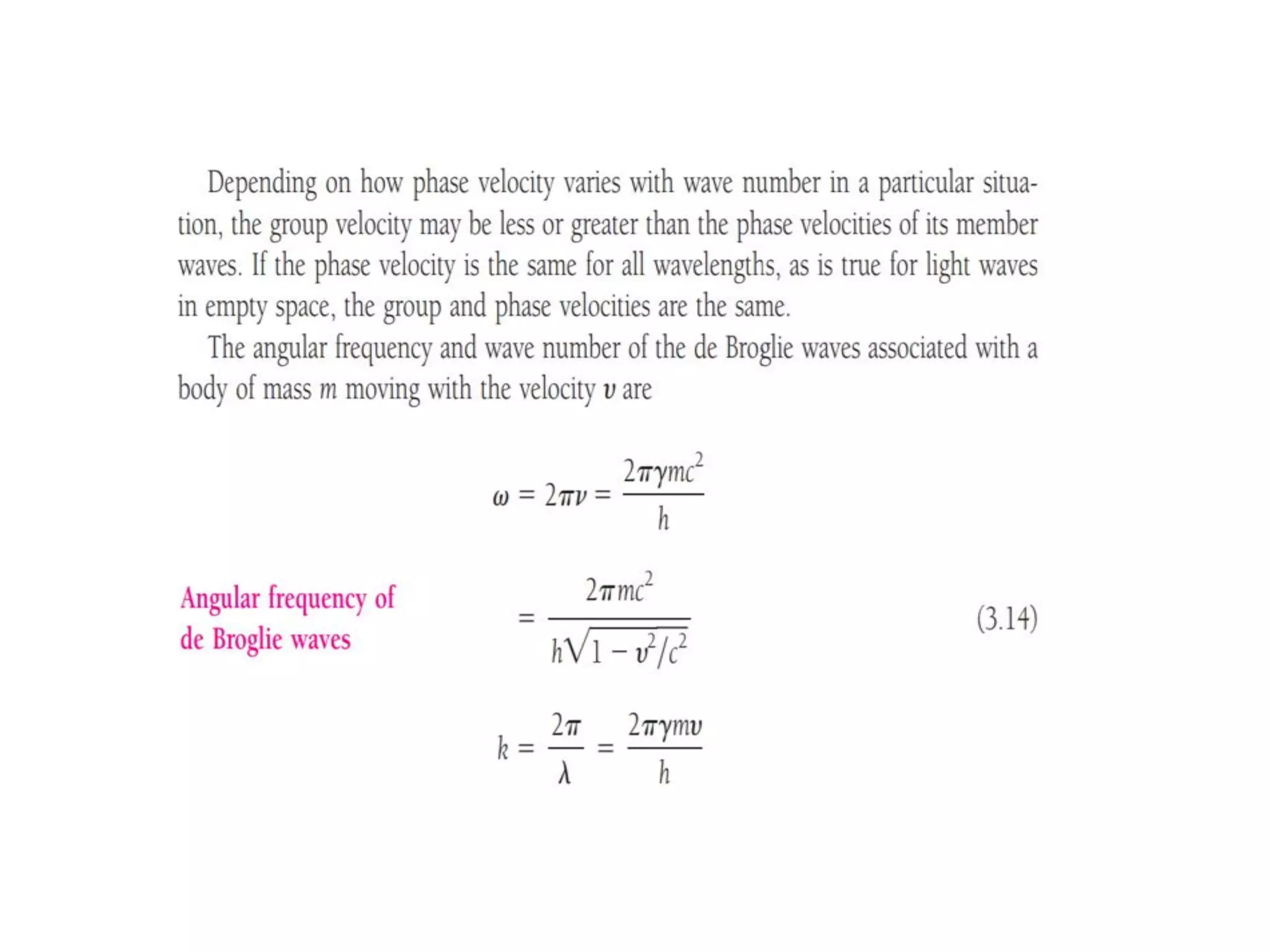
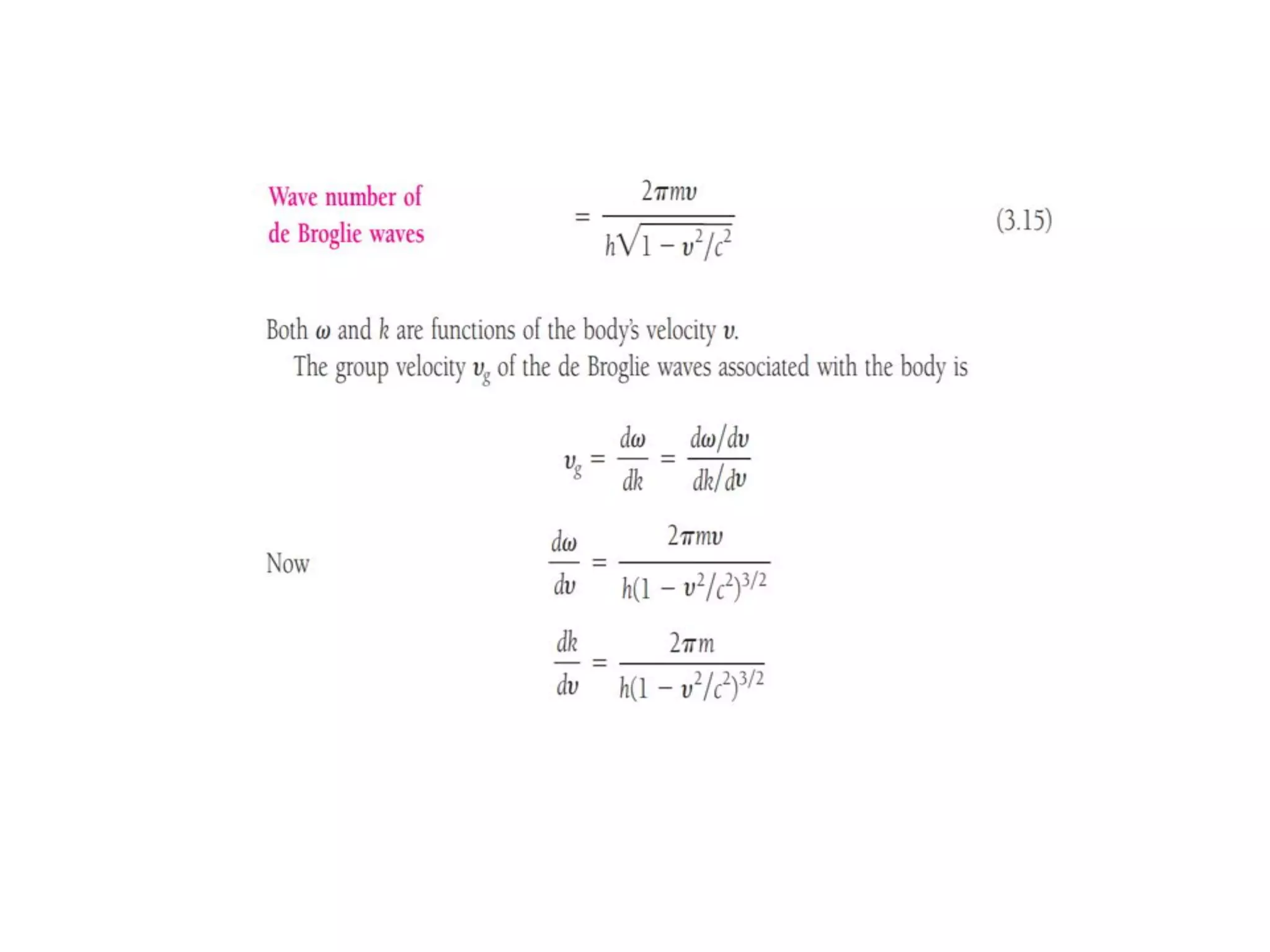
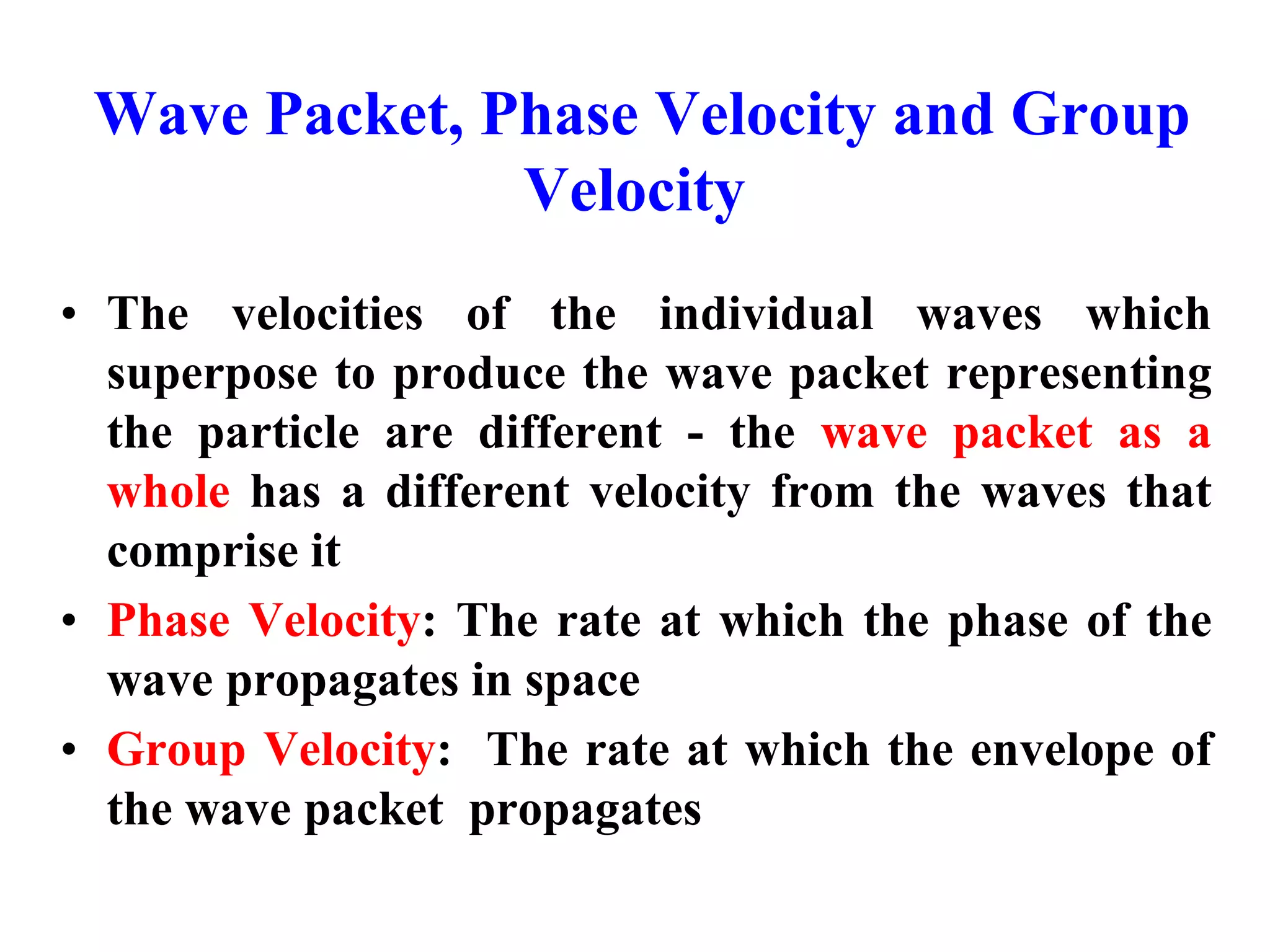
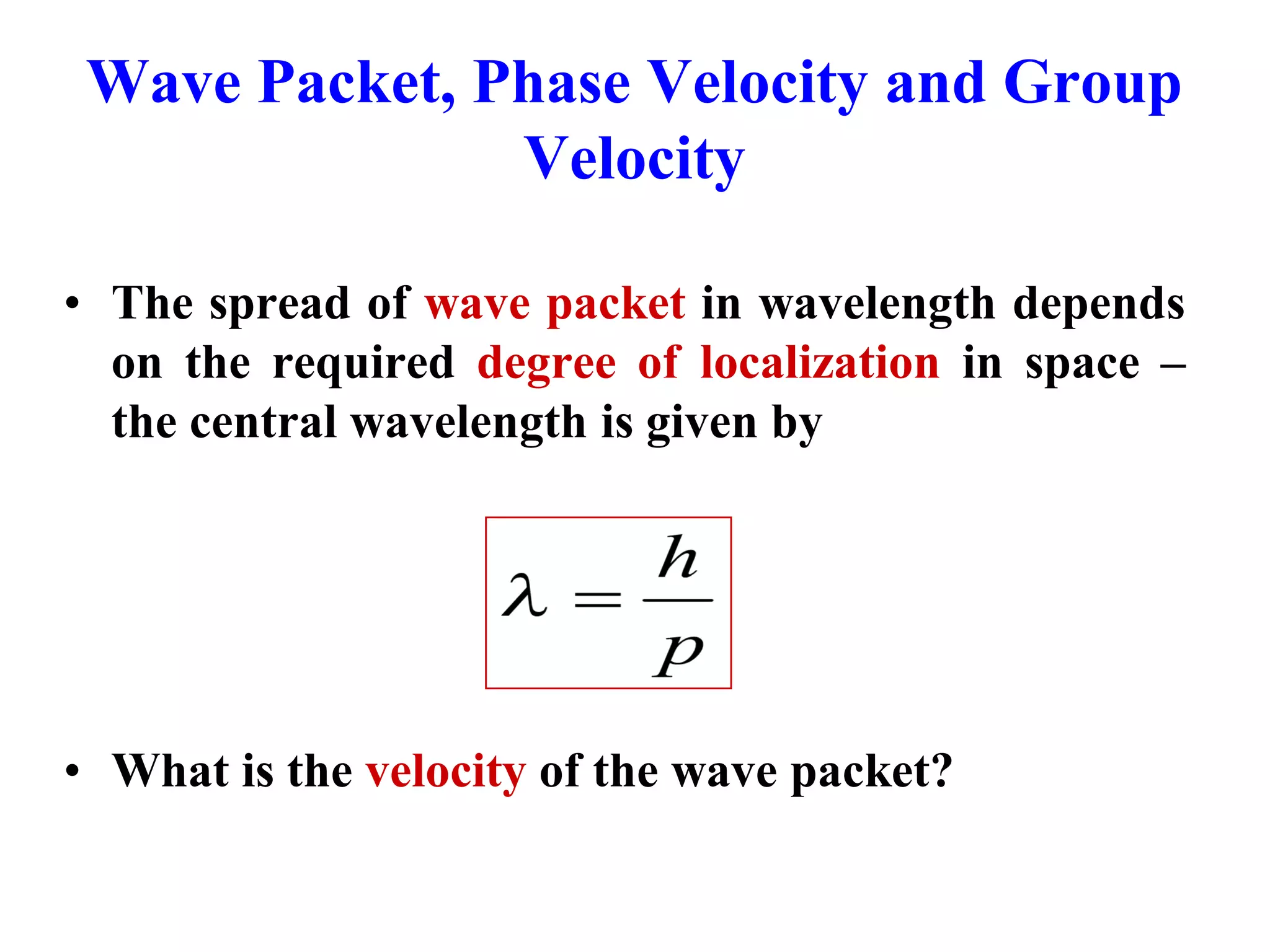
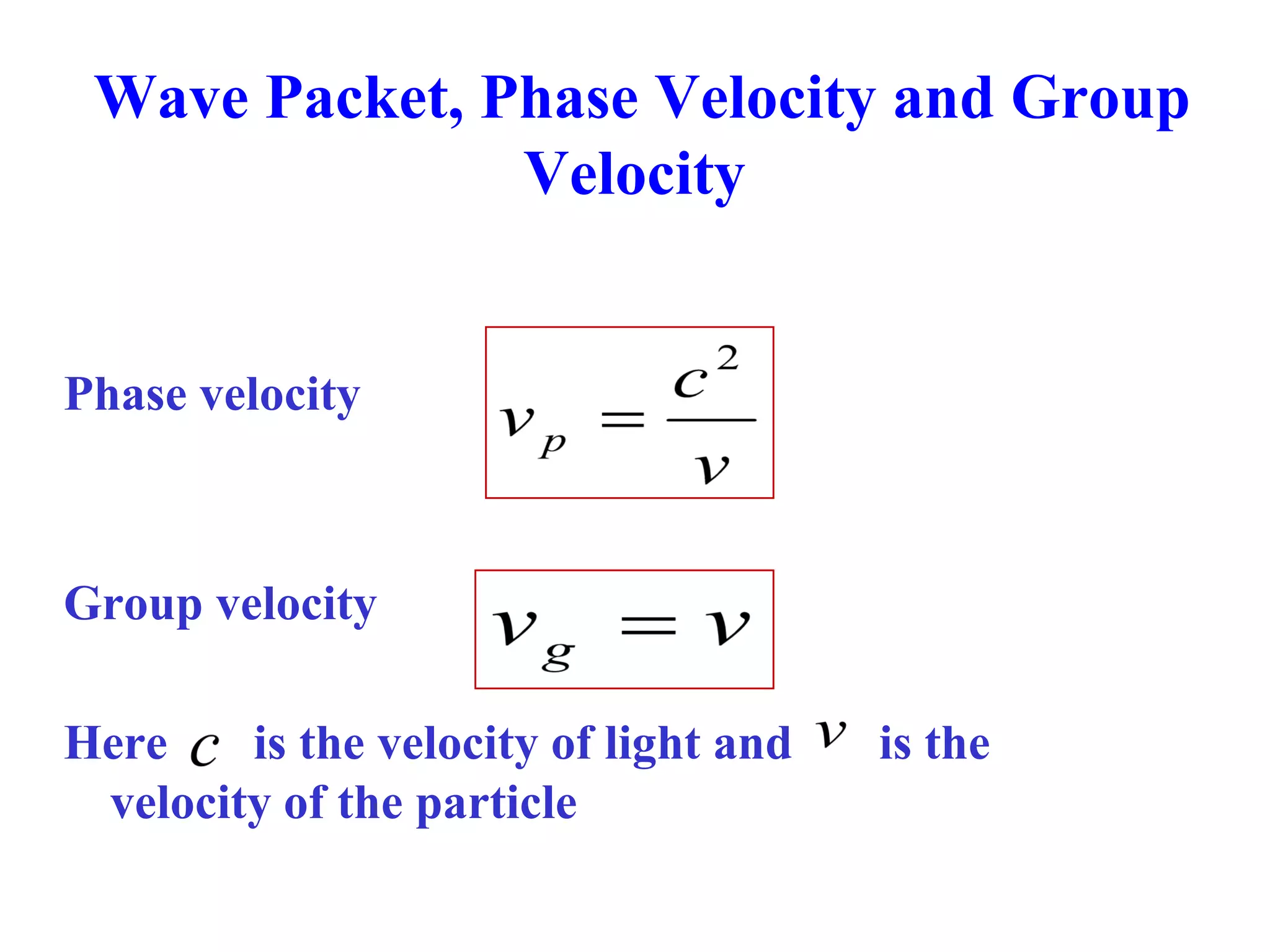
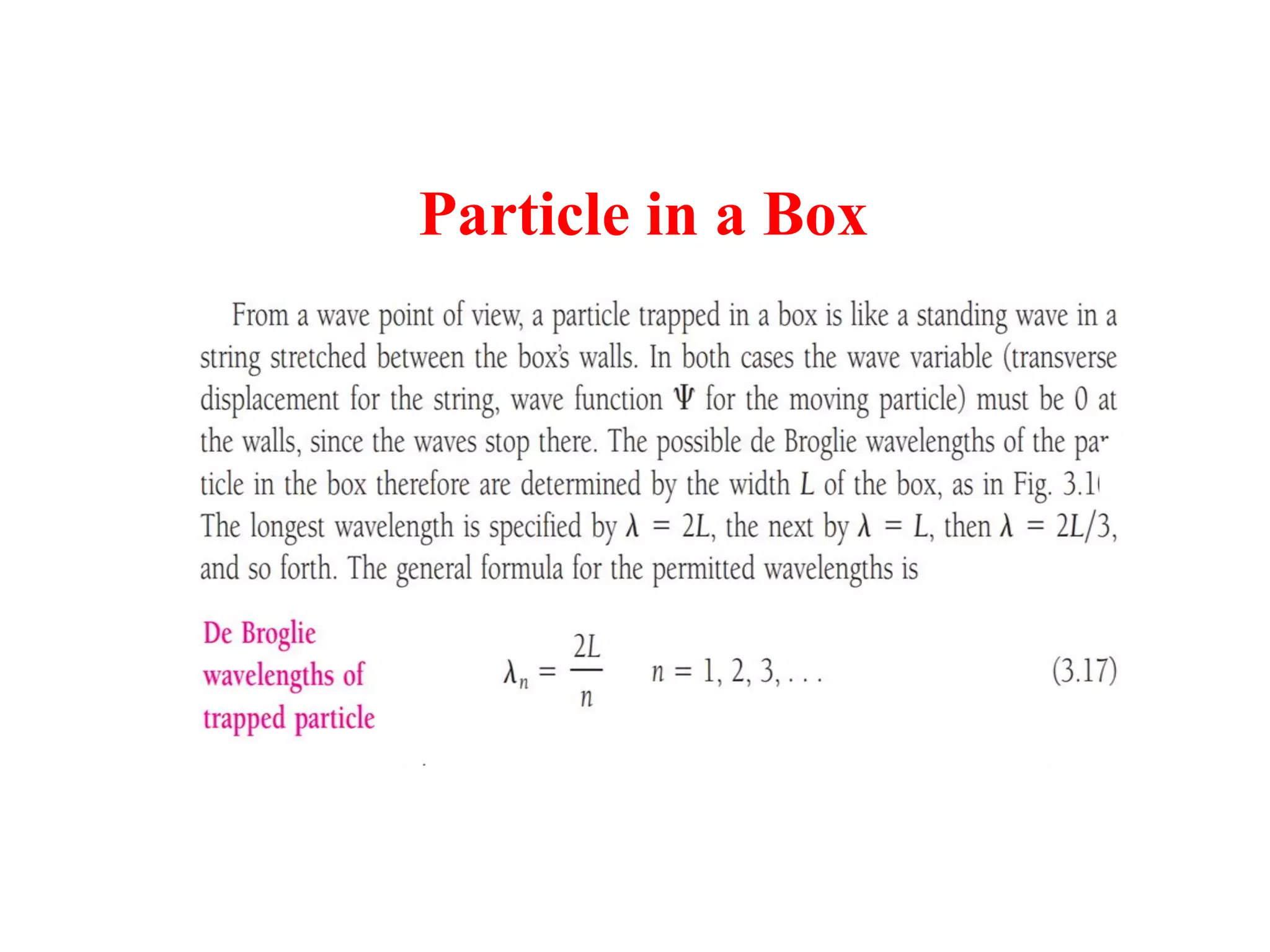
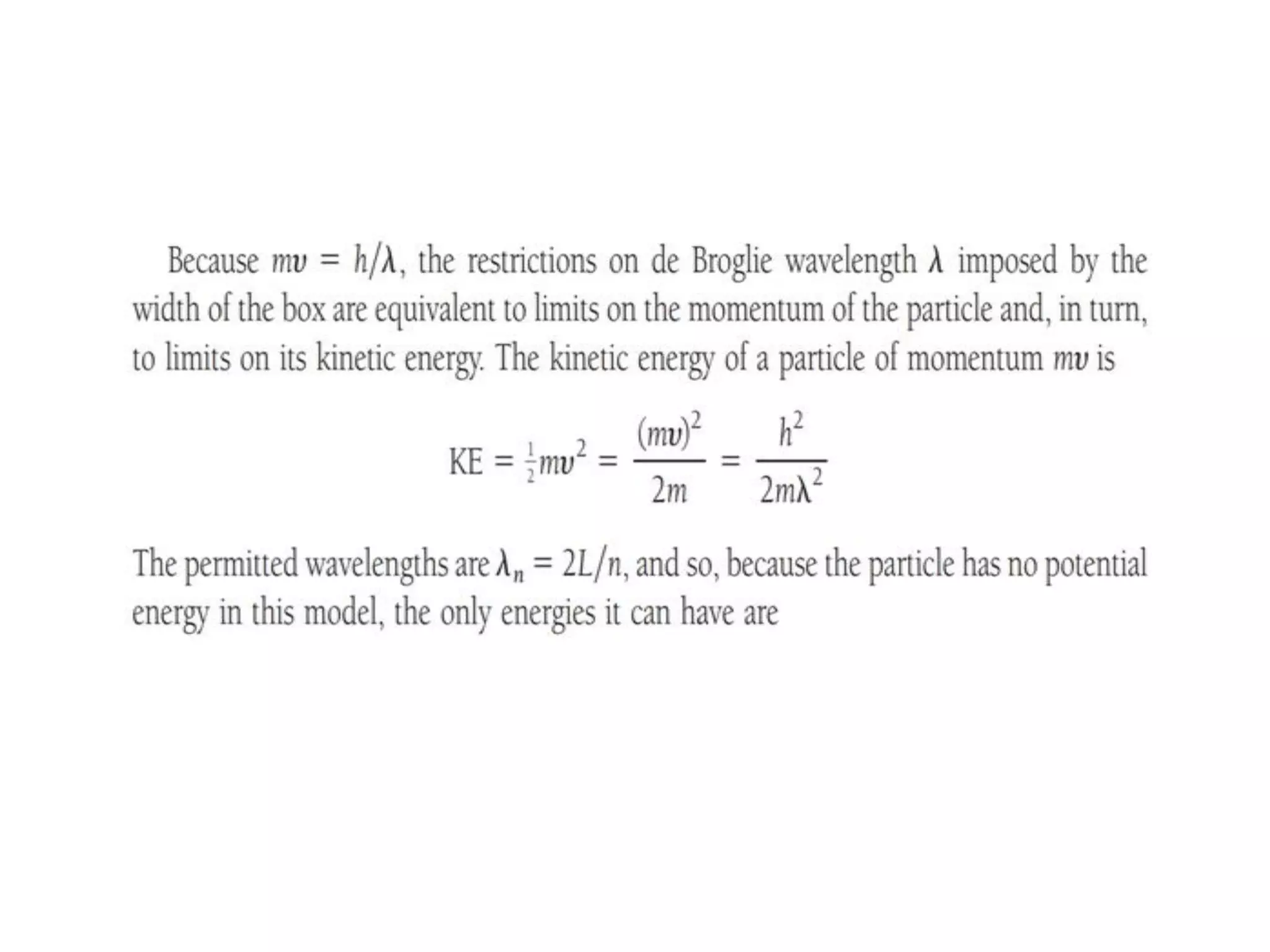
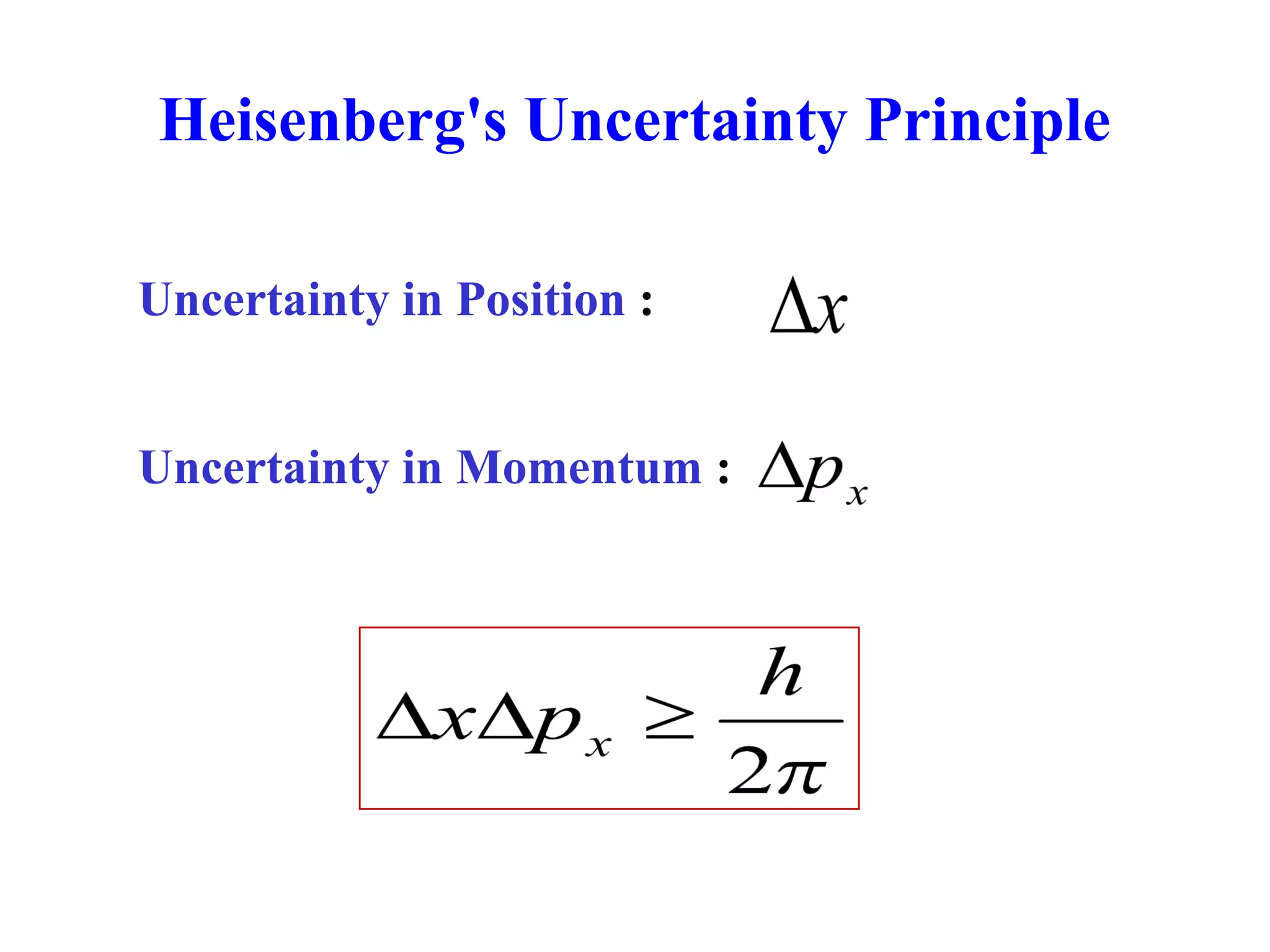
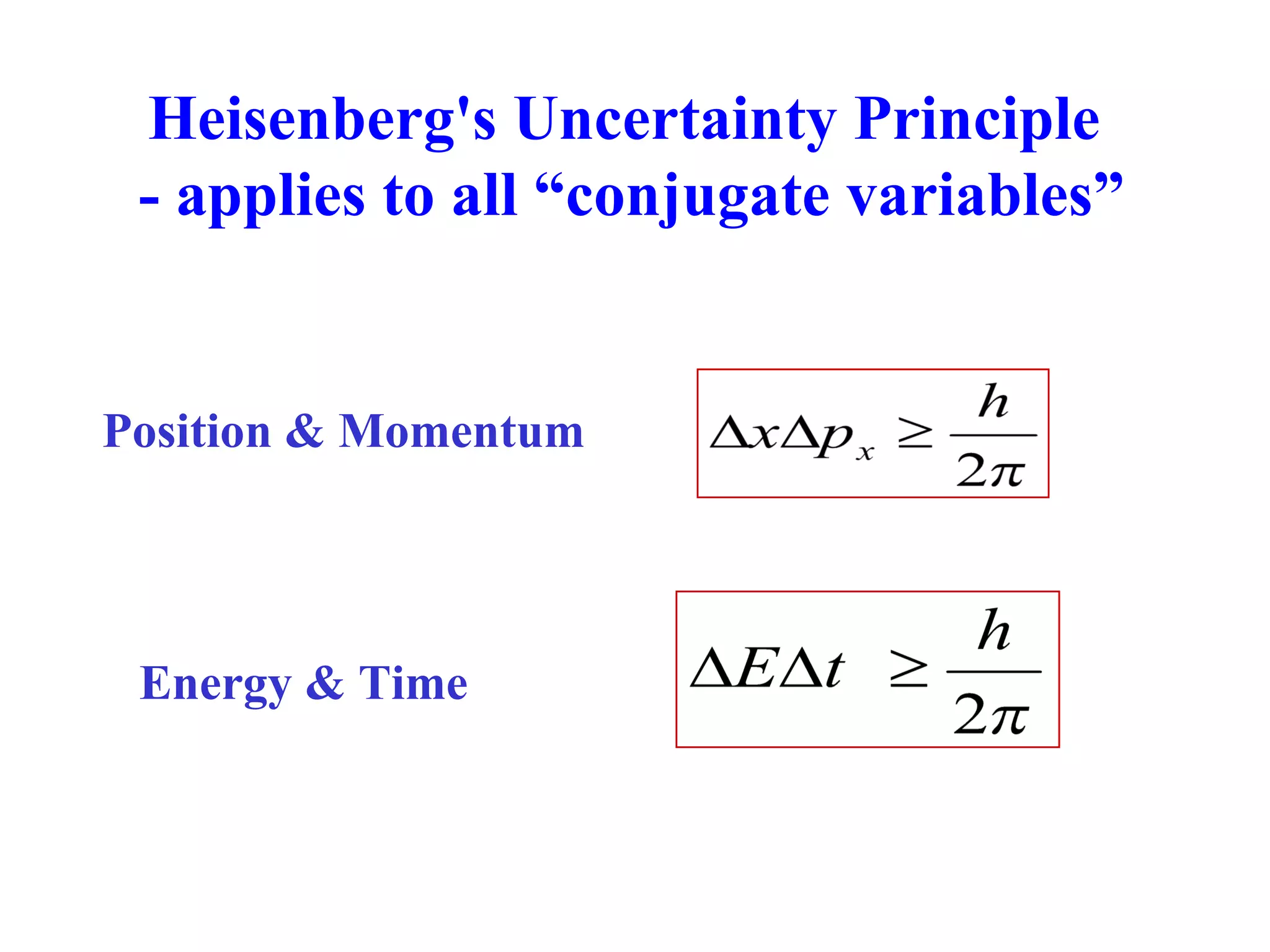
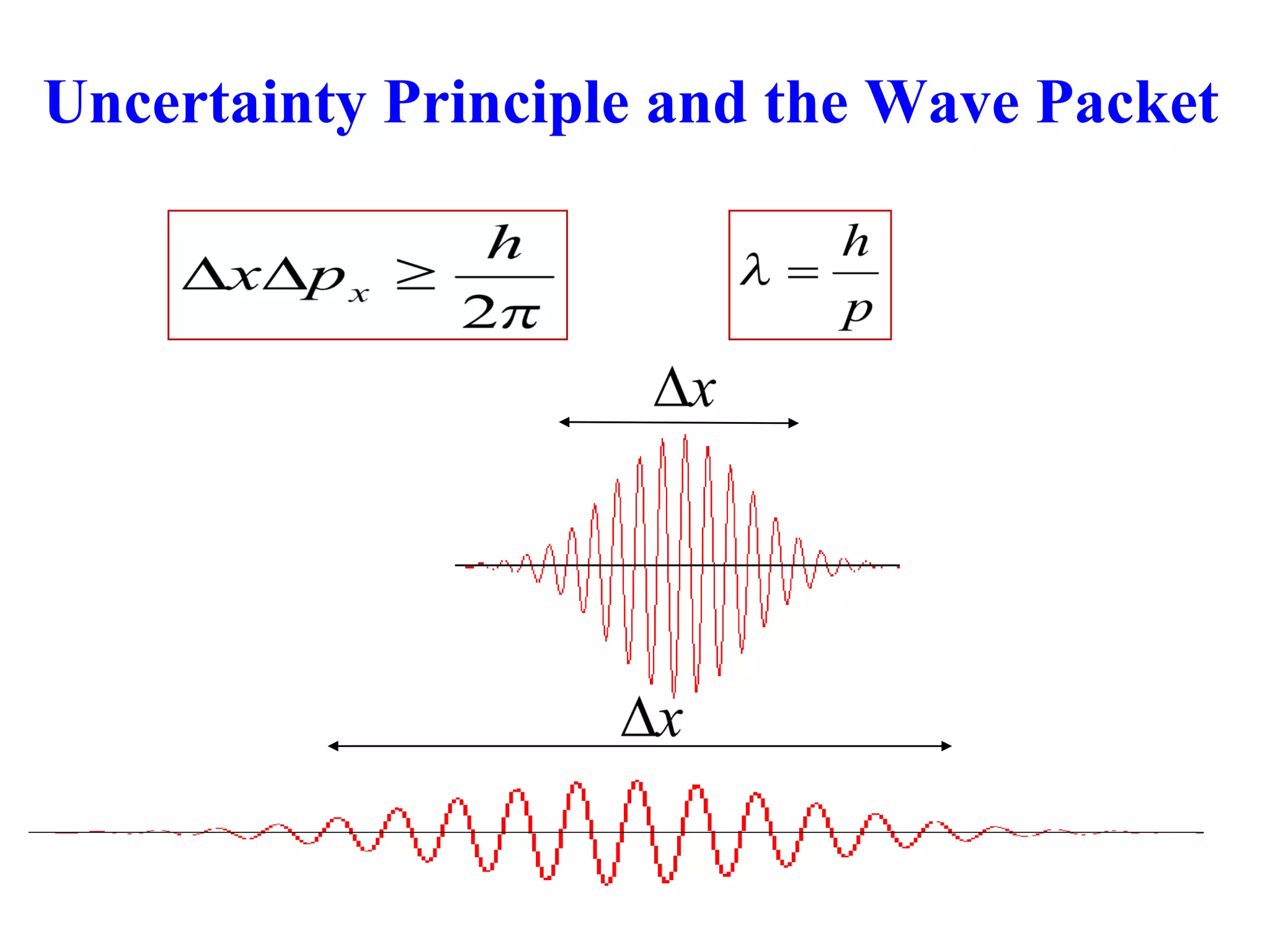
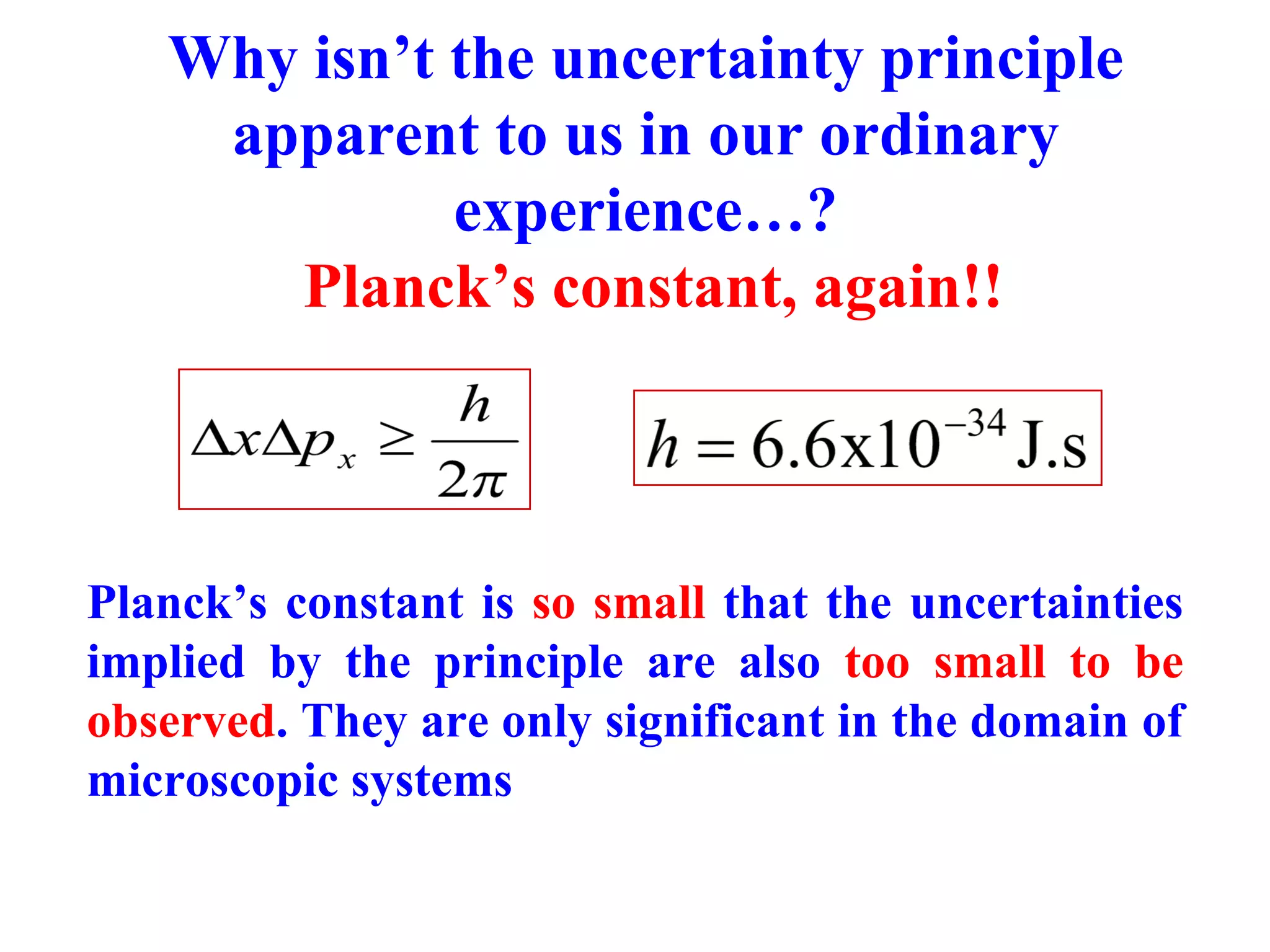
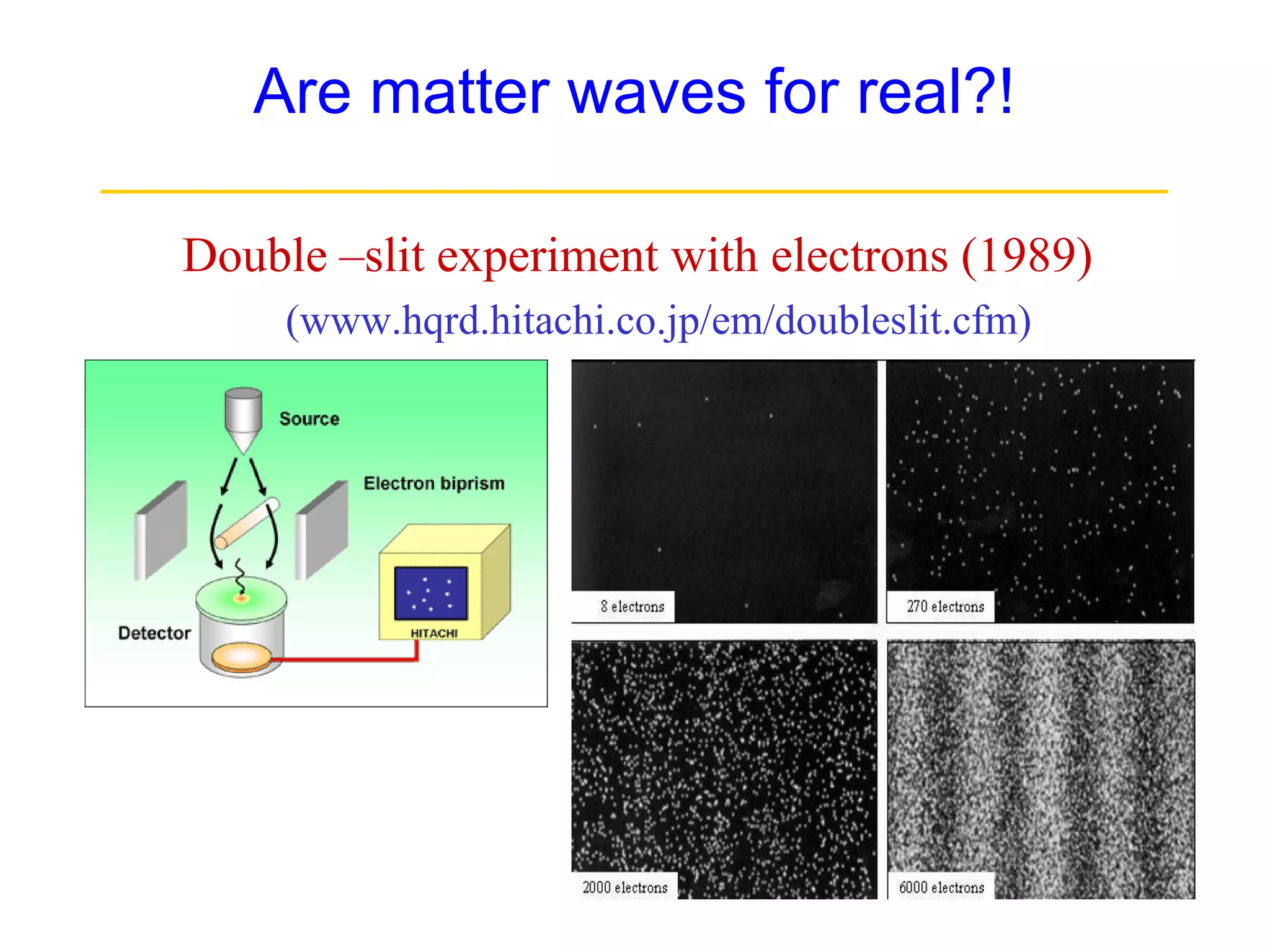
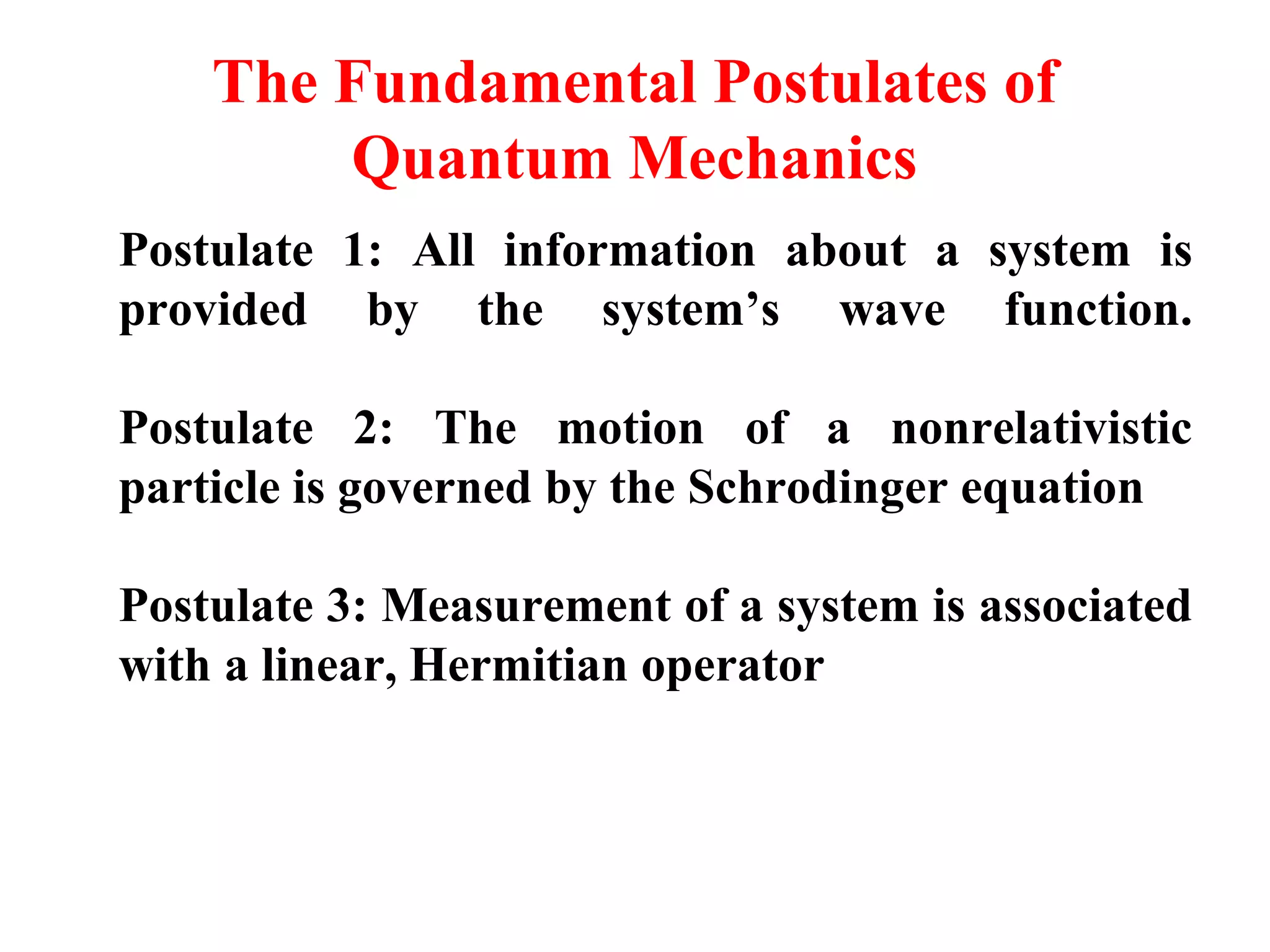
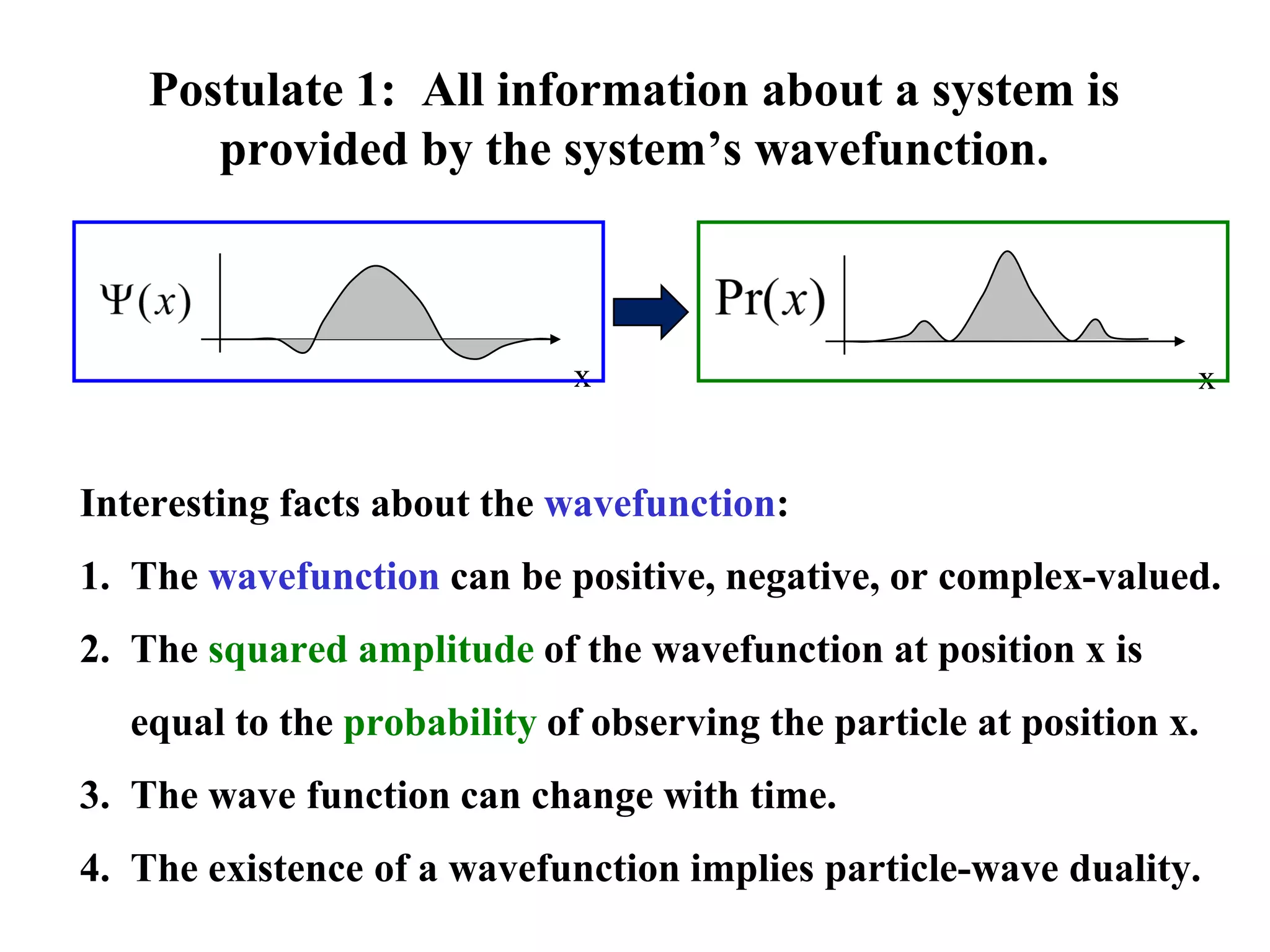
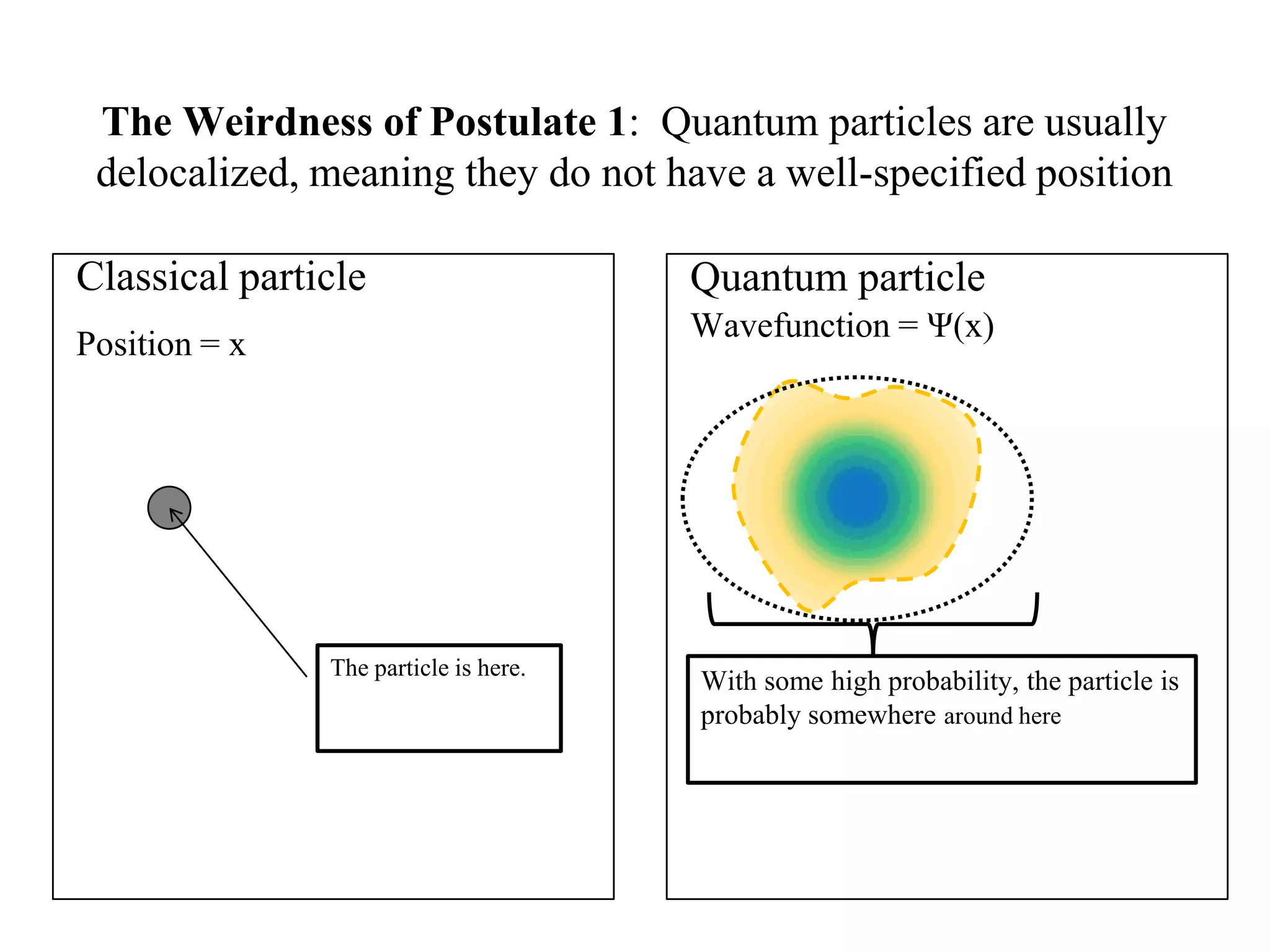
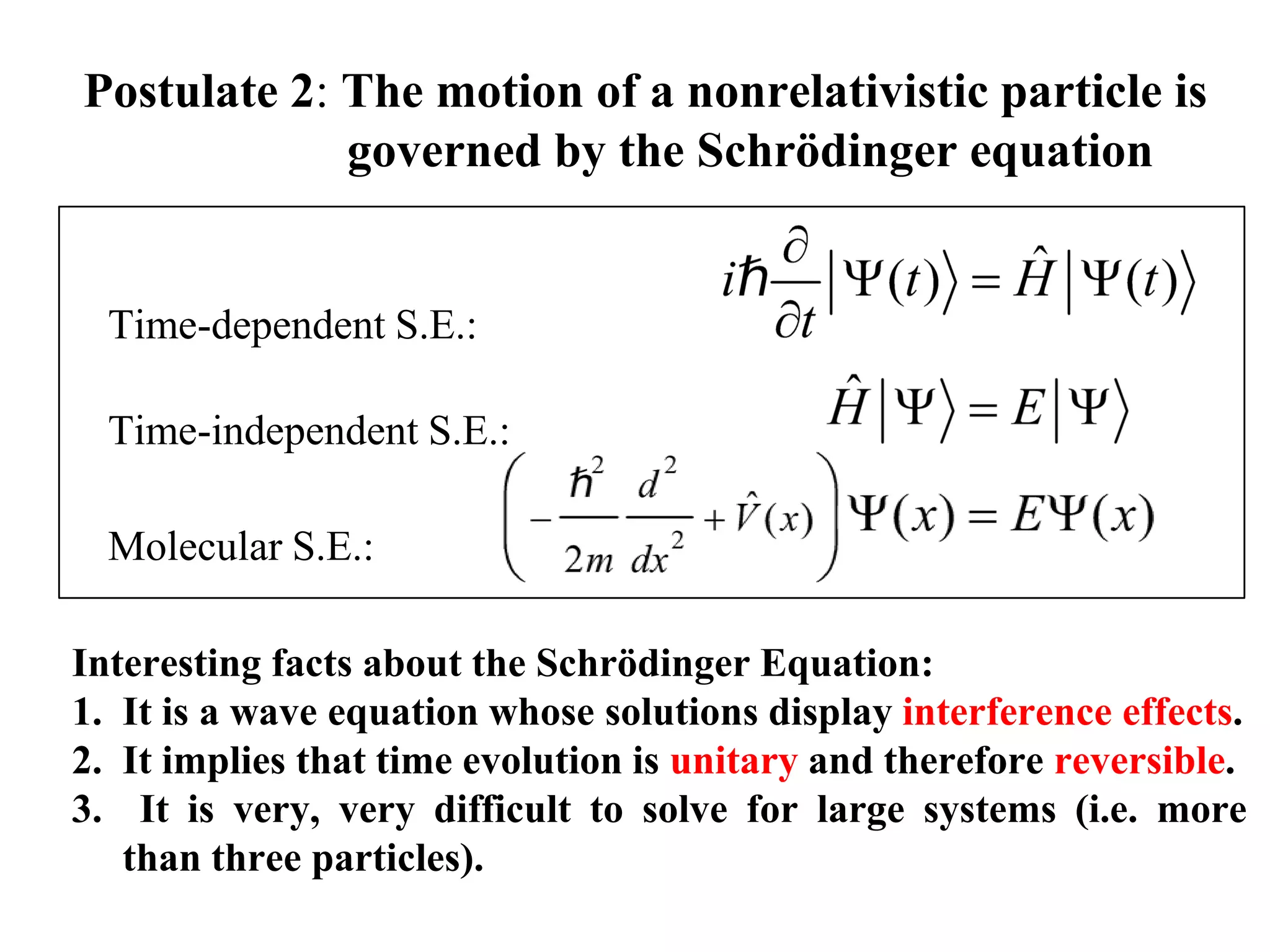
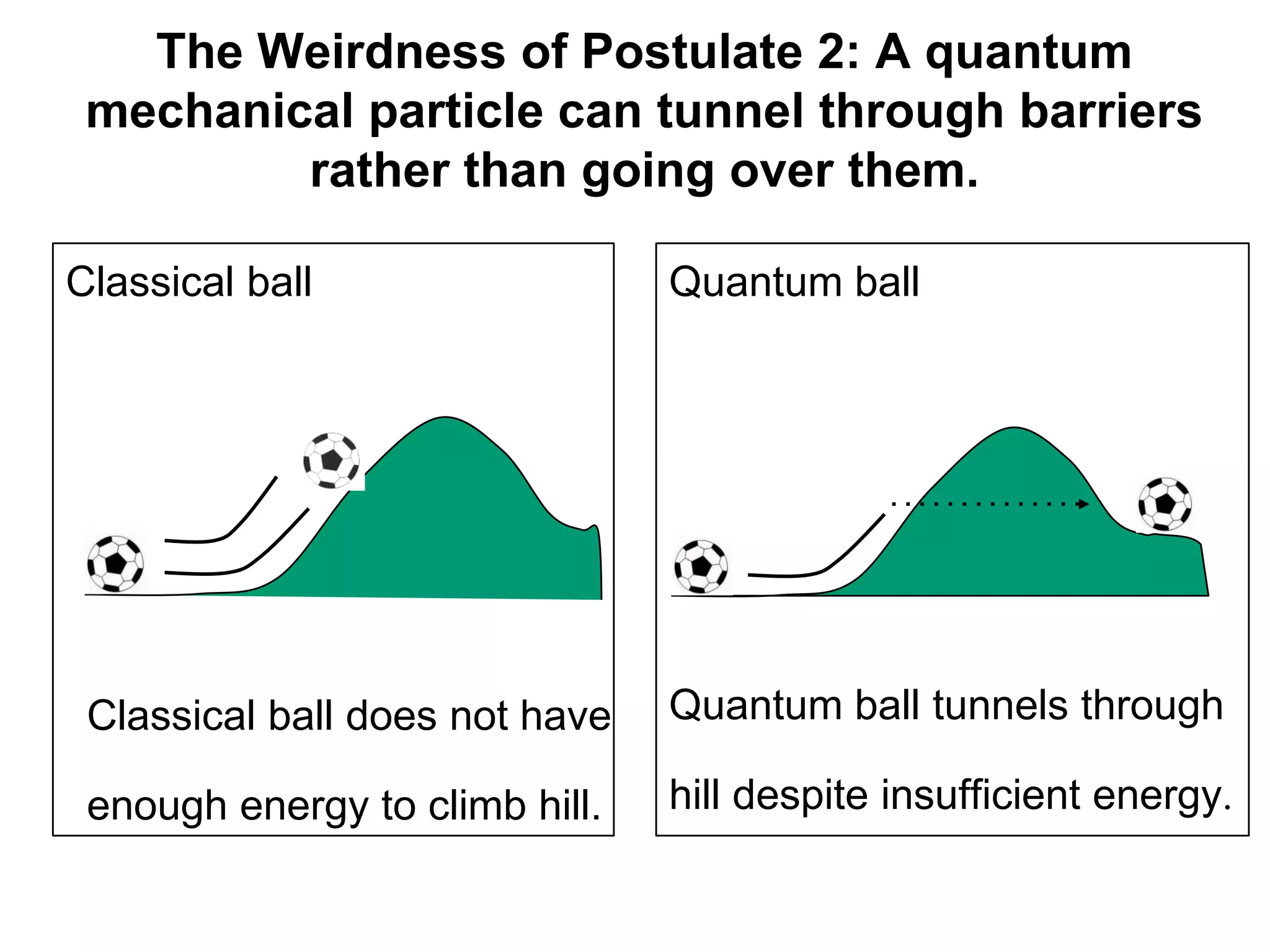
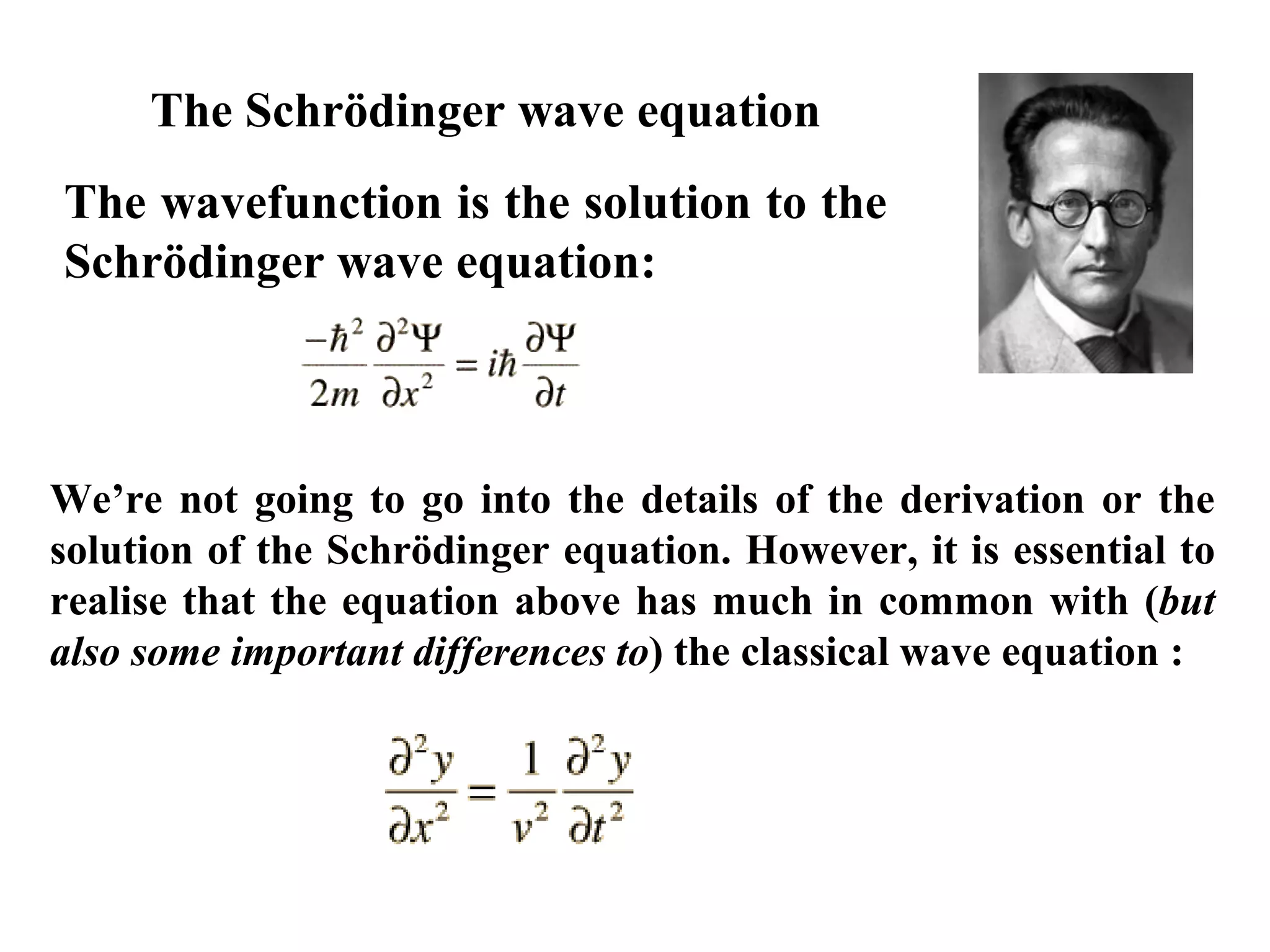
The document discusses the development of quantum mechanics and wave-particle duality, including de Broglie's hypothesis that all matter has an associated wave nature. It covers key concepts like the wave function, wave packets, Heisenberg's uncertainty principle, and the Schrödinger equation governing the motion of quantum particles. Experimental evidence for matter waves is also discussed, showing that even large molecules can exhibit wave-like behavior.