This document provides an introduction to quantum mechanics concepts including:
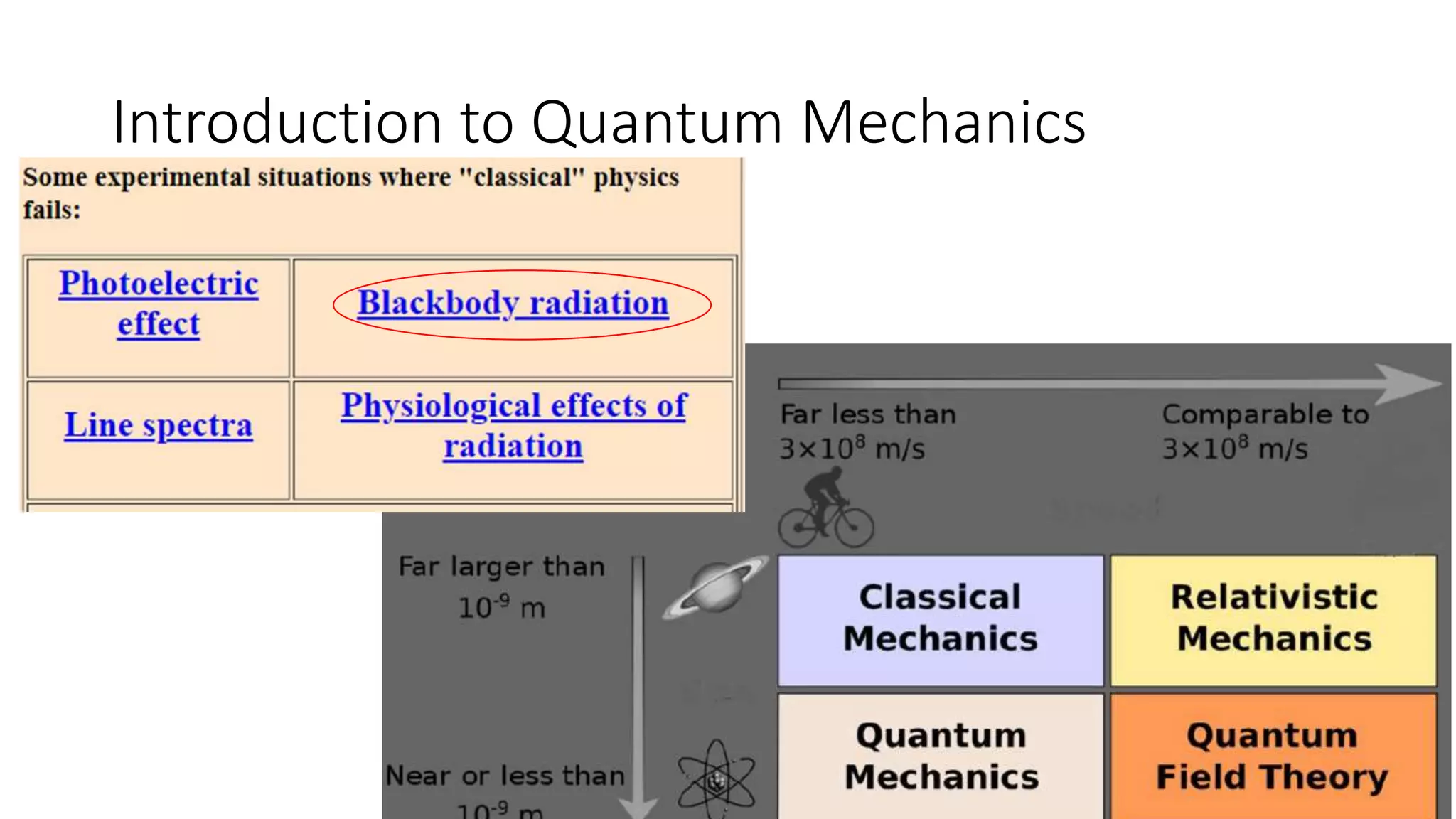
- Quantum mechanics describes nature at small scales where classical physics is insufficient. Pioneers who established the foundations of quantum mechanics are mentioned.
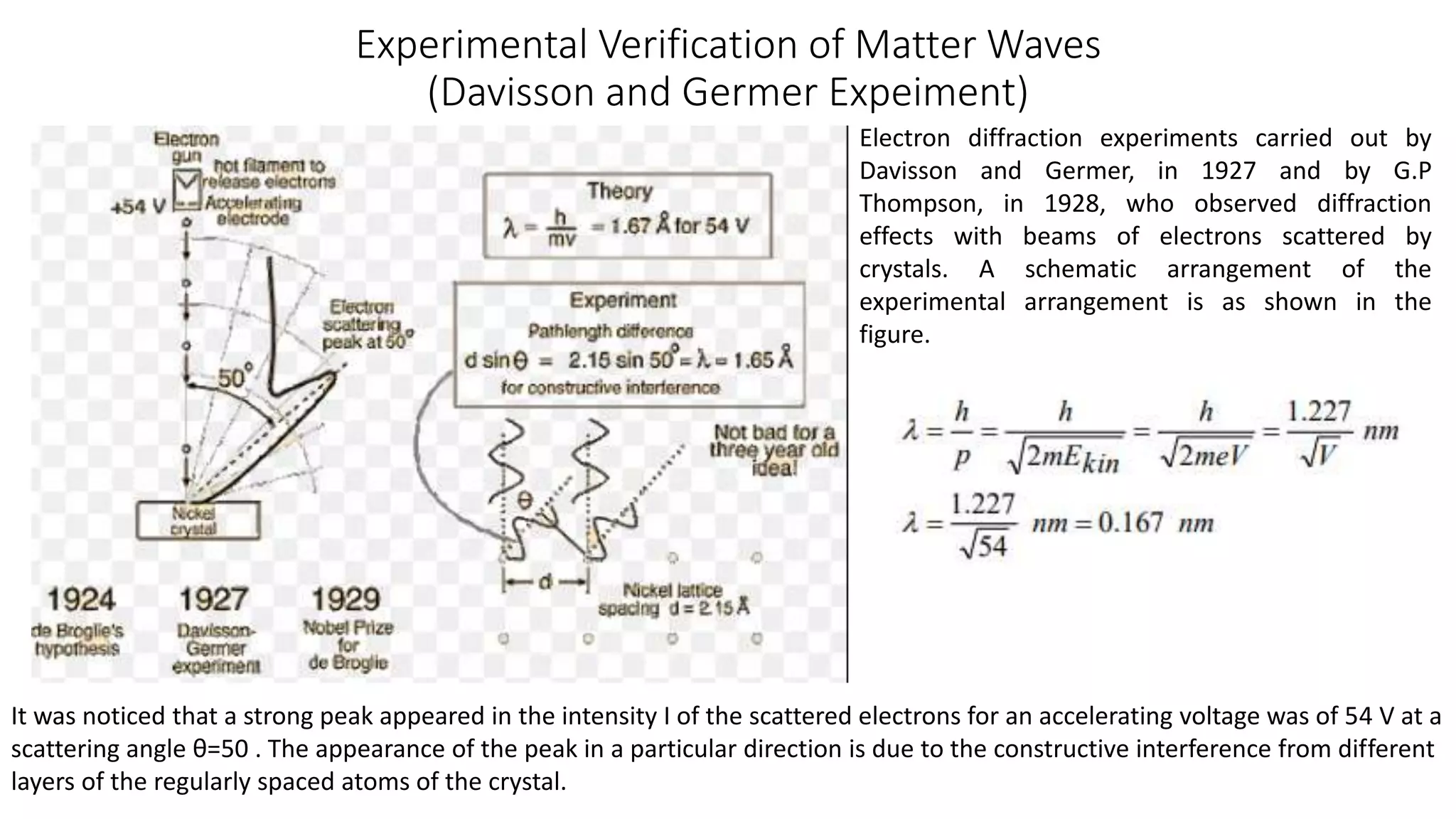
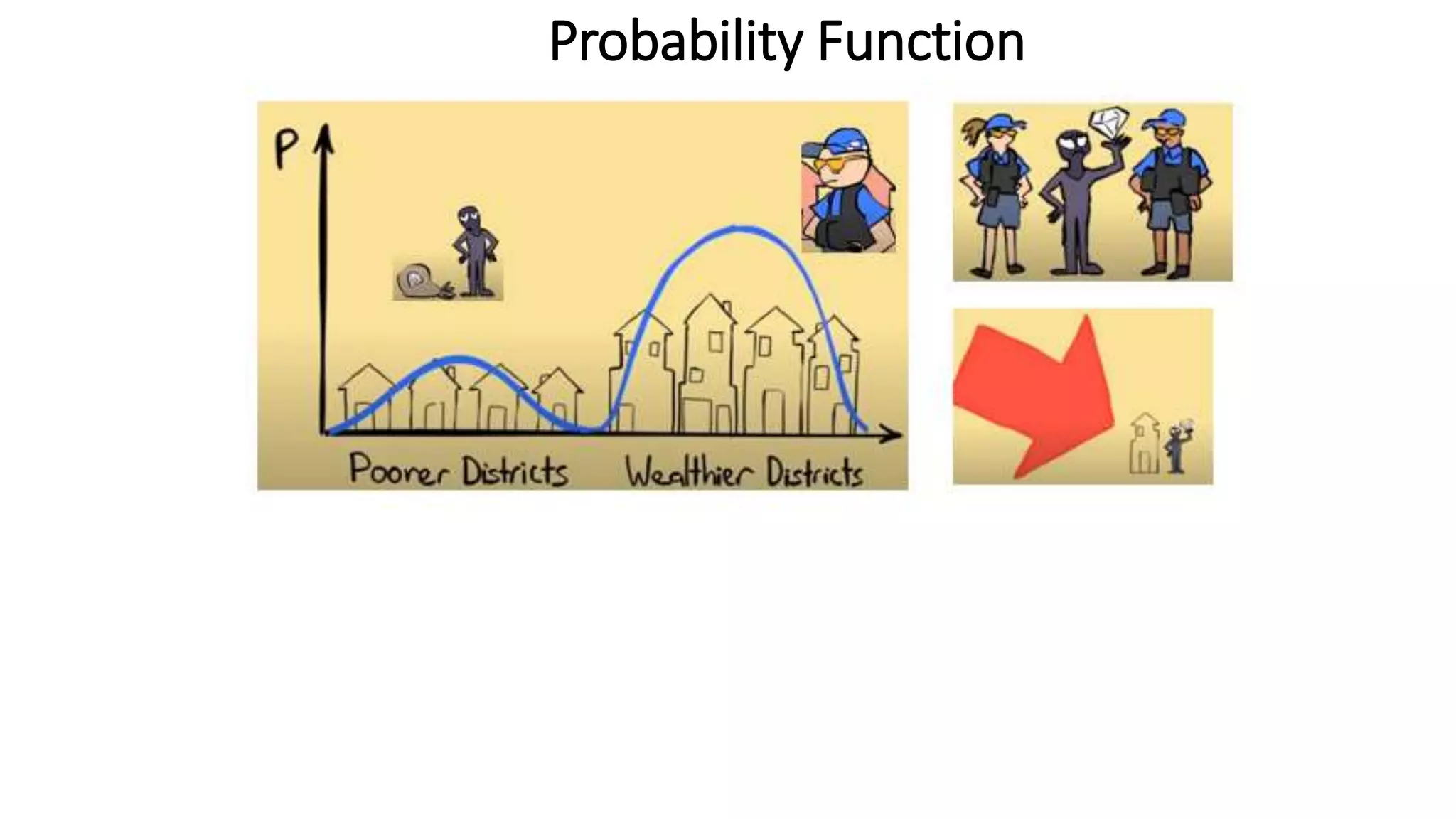
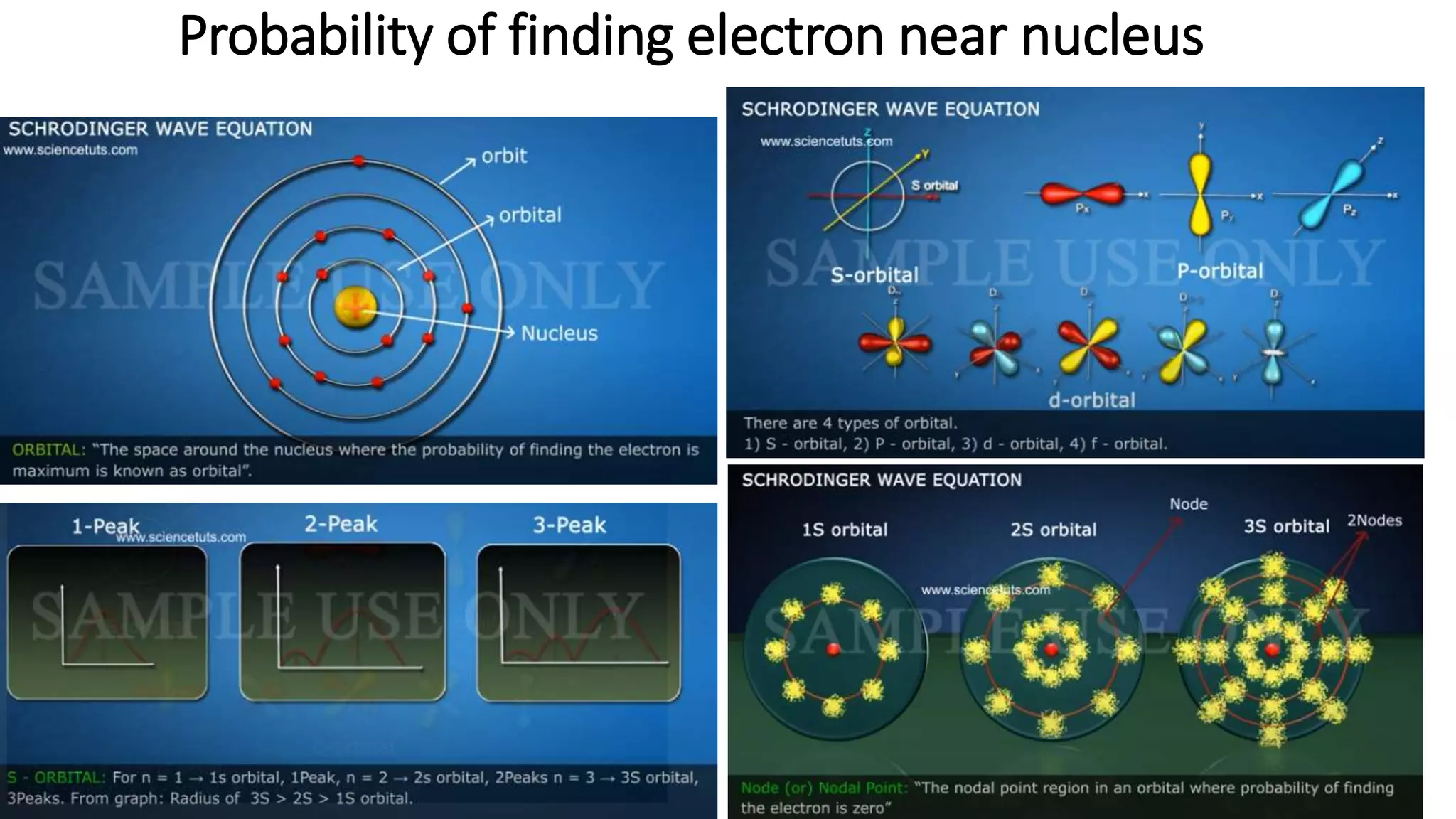
- Key concepts are introduced such as wave-particle duality, matter waves, Heisenberg's uncertainty principle and its application to electrons not existing in atomic nuclei.
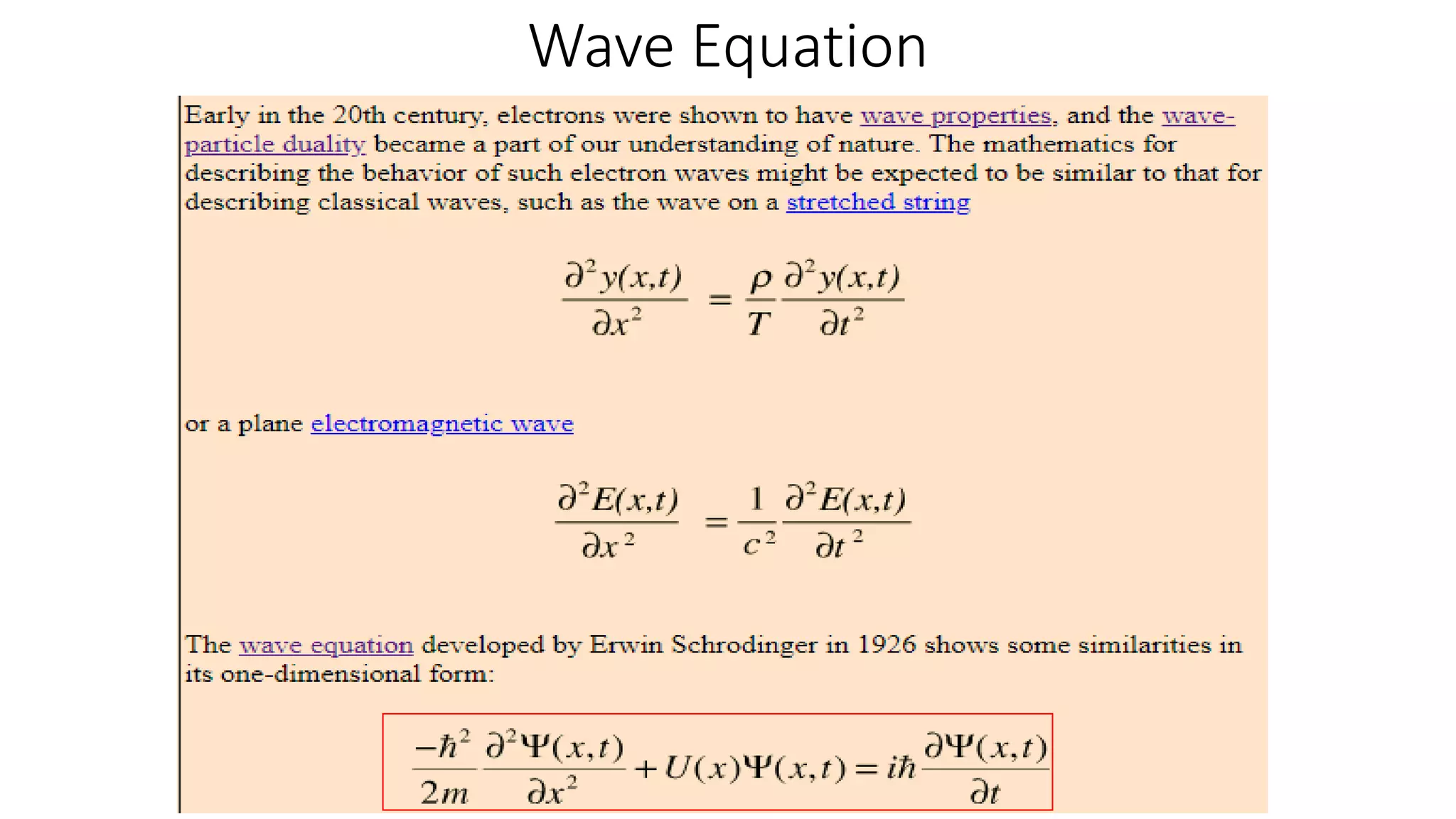
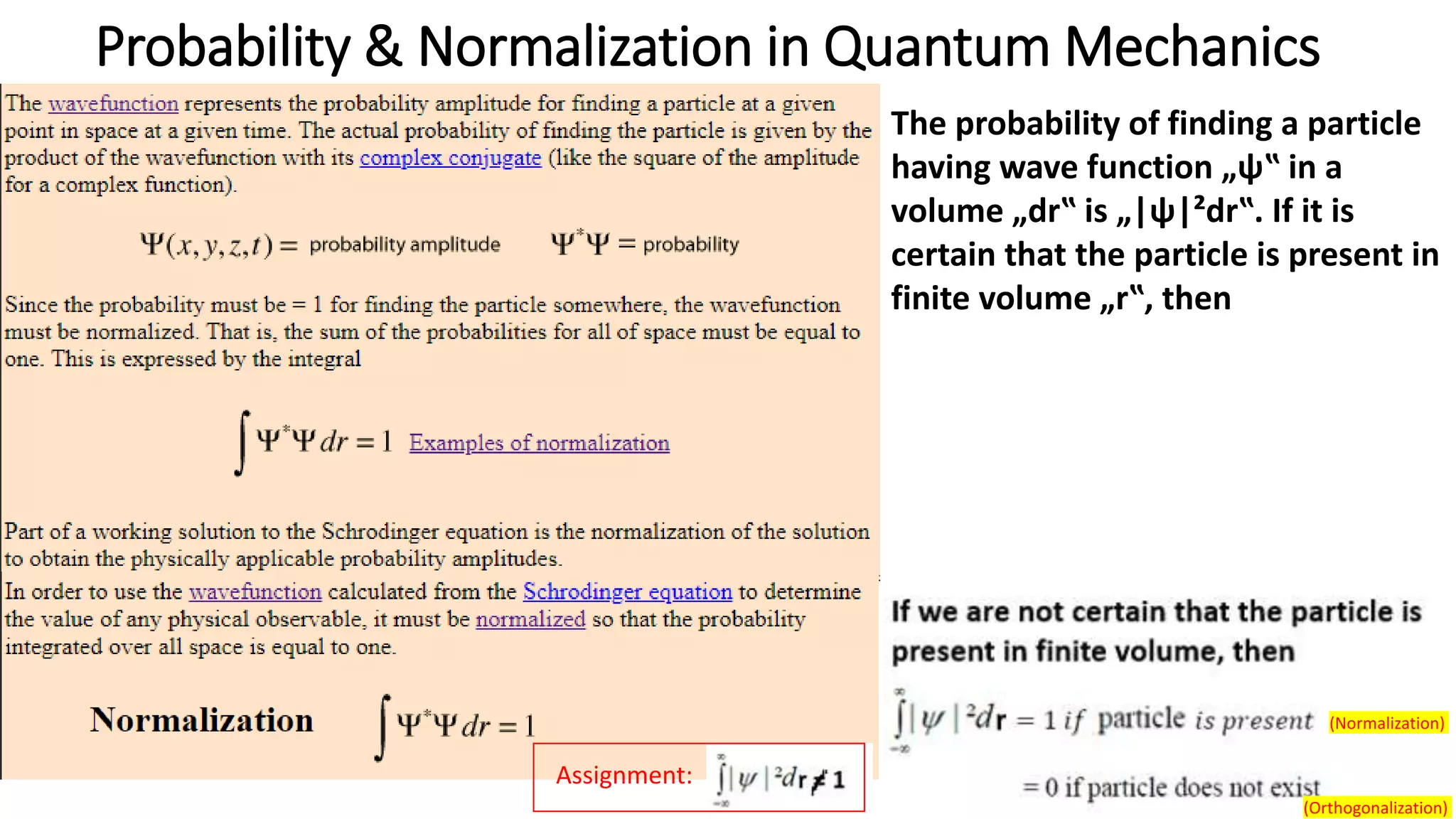
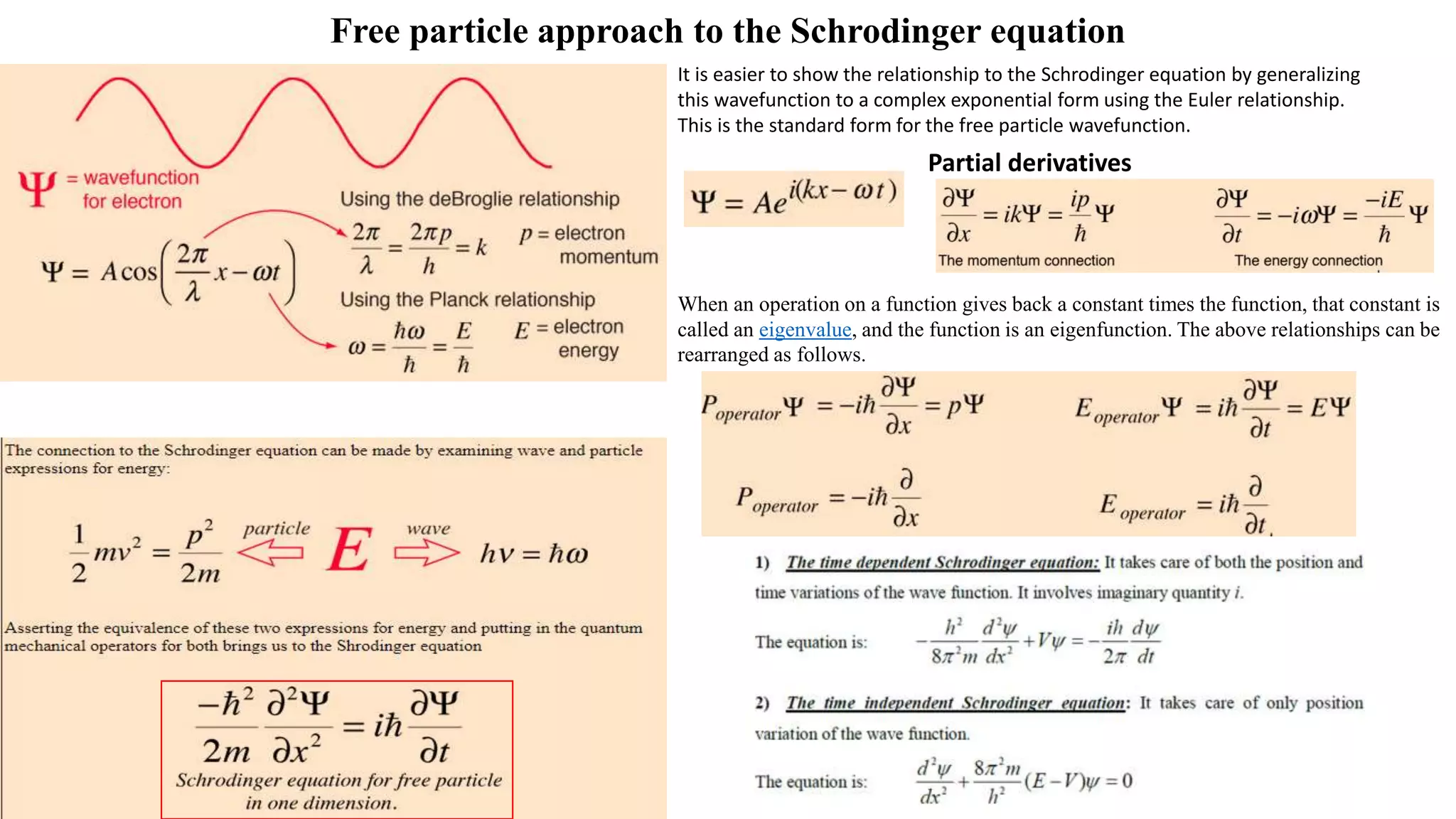
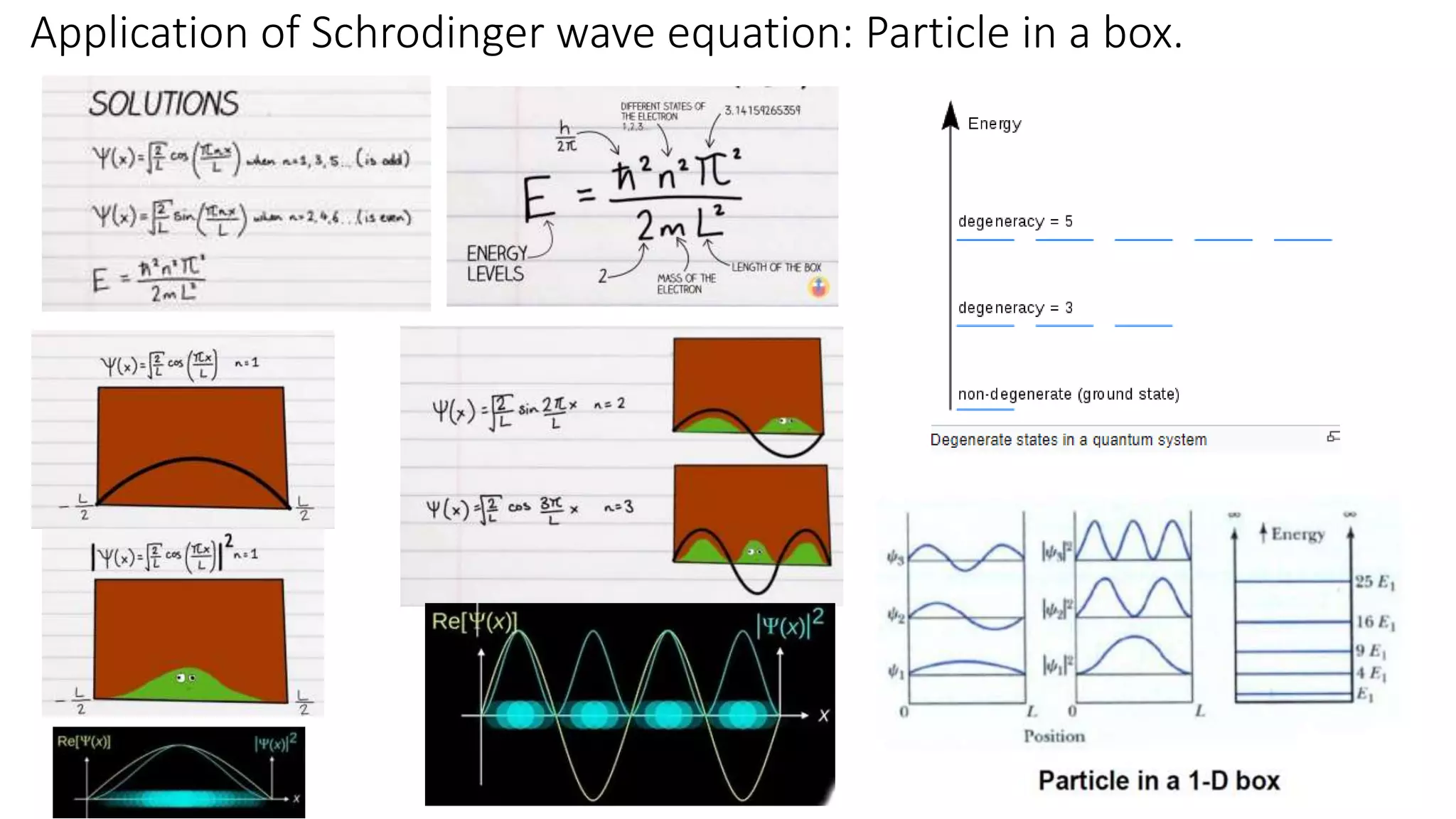
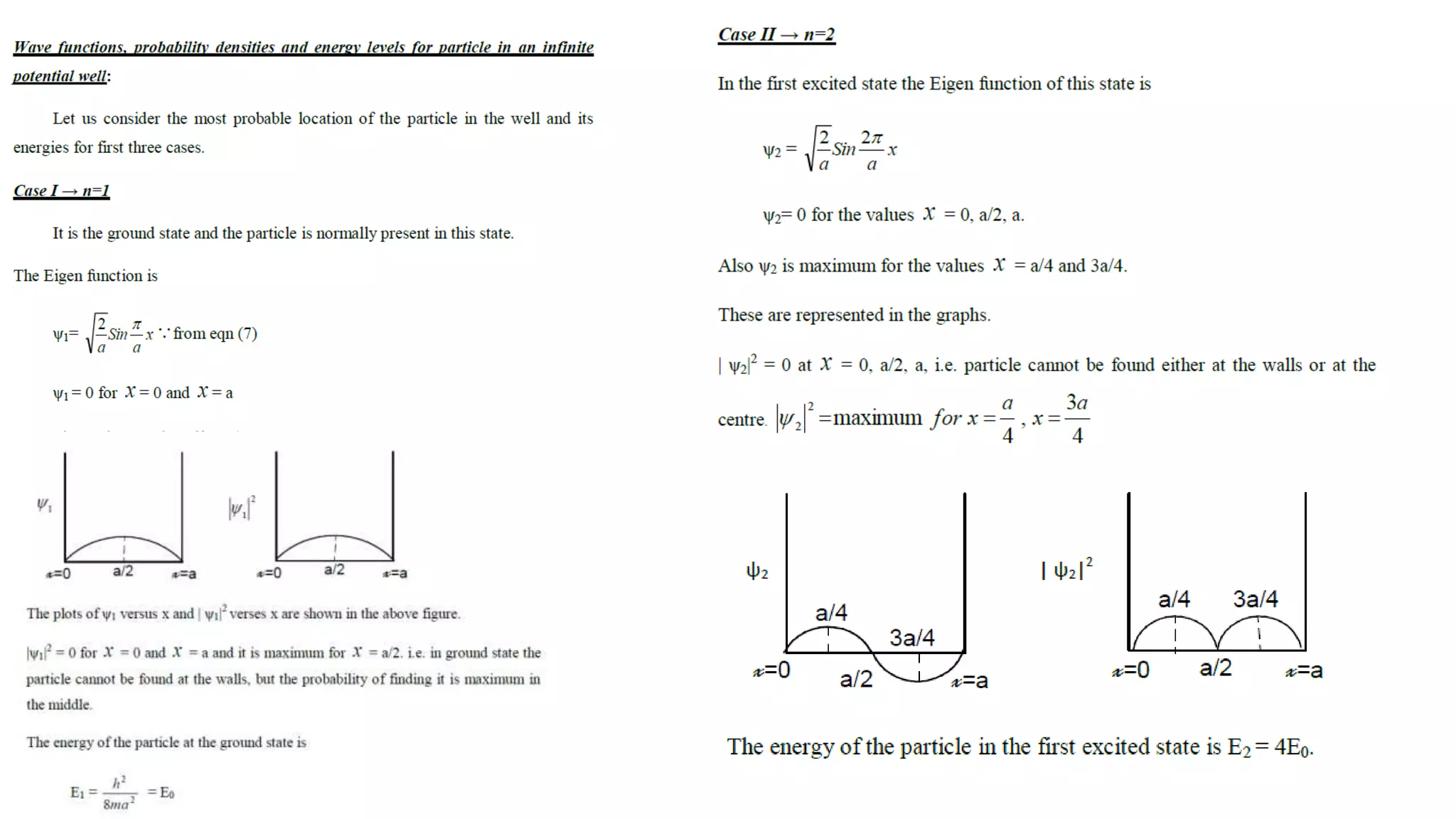
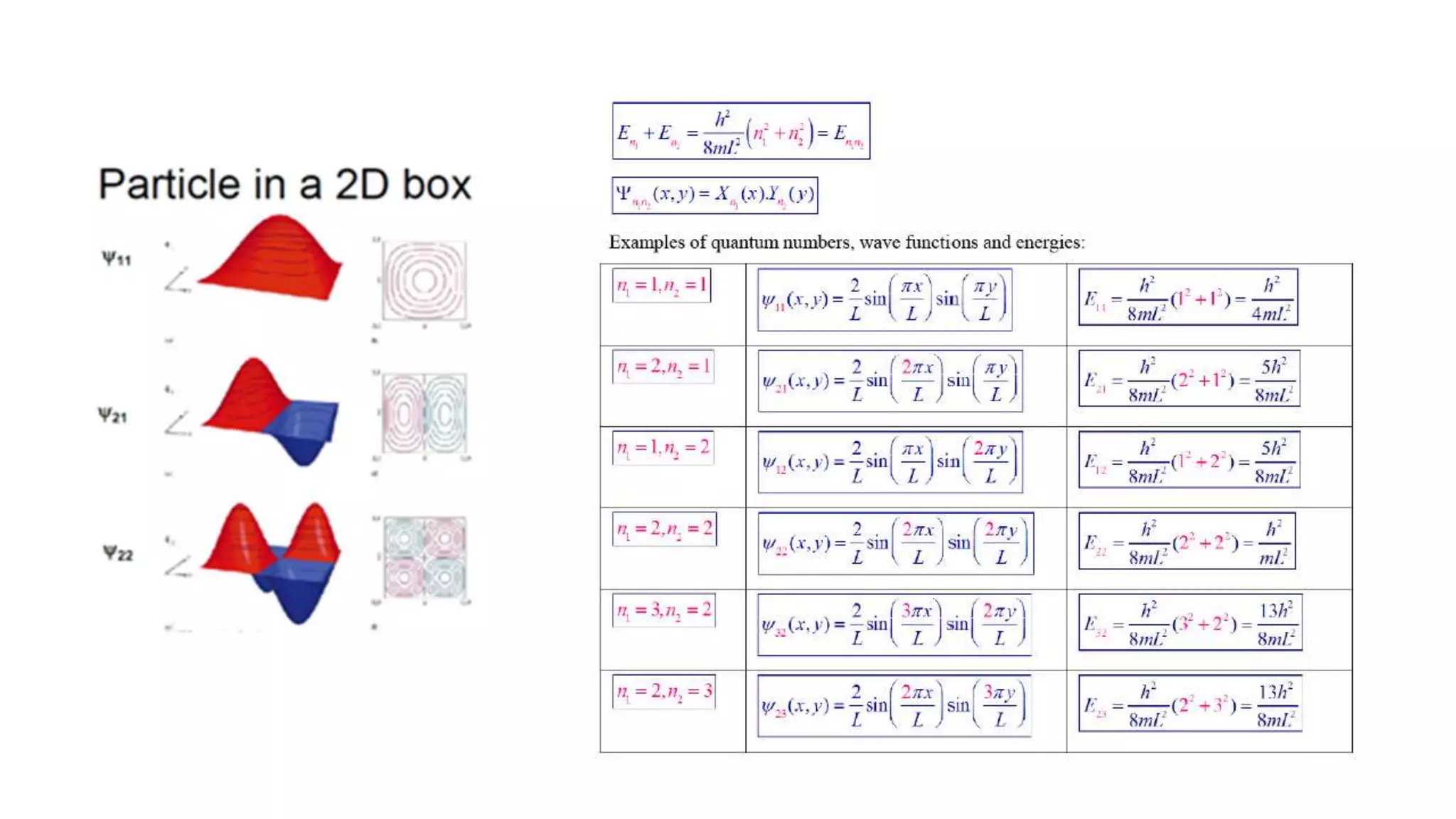
- The Schrodinger wave equation is derived and applied to problems such as a particle in an infinite potential well to solve for energy eigenstates and eigenvalues.