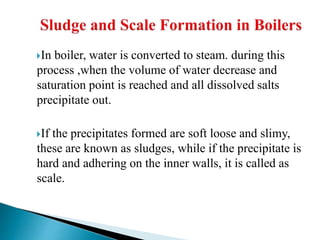
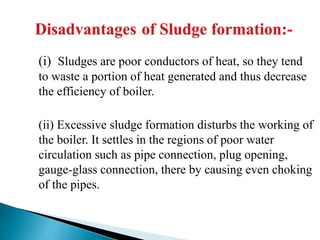
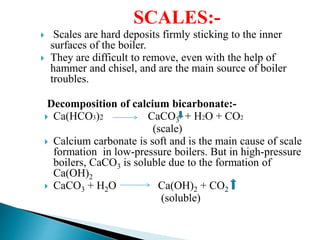
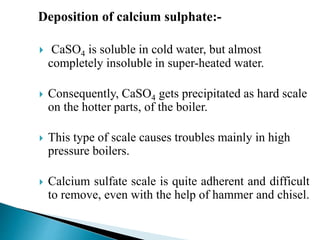
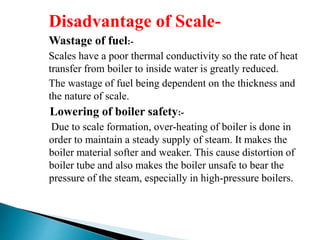
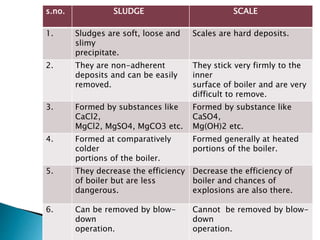
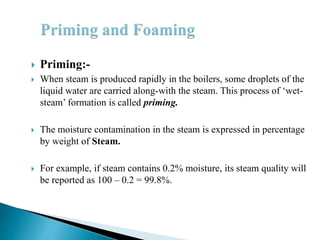
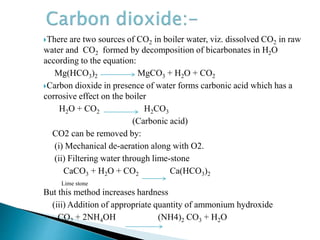
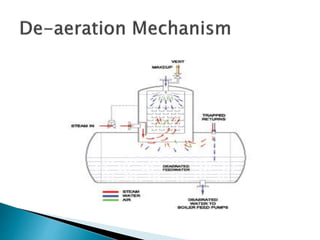
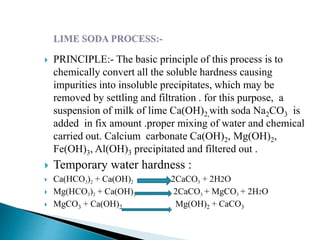
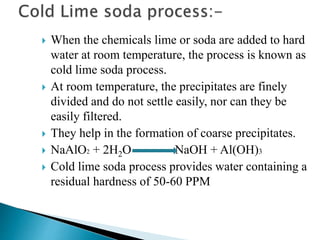
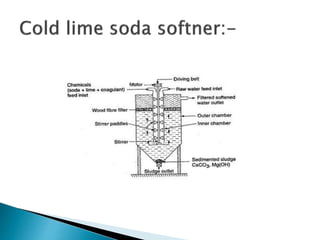
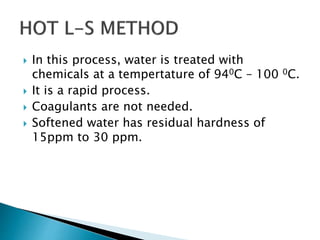
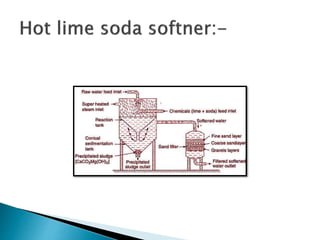
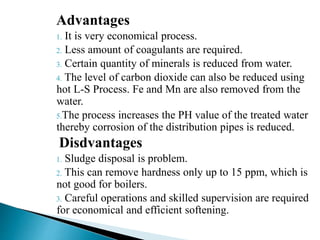
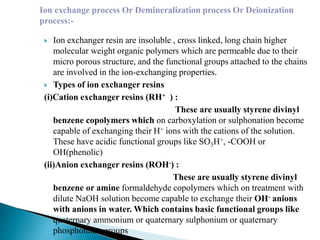
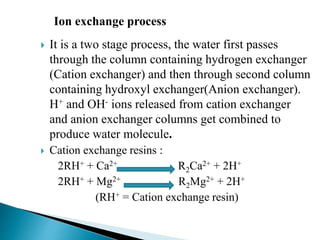
The document discusses boiler water problems and softening methods. It describes how excess impurities in boiler feed water can cause issues like sludge and scale formation, priming and foaming, carry over, boiler corrosion, and caustic embrittlement. It defines sludge and scale, explains how they form and their impacts. Methods to prevent or remove sludge and scale are provided. Causes and controls of priming, foaming, and carry over are also outlined. The document then discusses various corrosion mechanisms and methods to prevent corrosion. Finally, it provides an overview of zeolite softening process.















![Dissolved oxygen:-
This is the most usual corrosion causing factor.
In boiler oxygen is introduce through the raw water
supply. Water usually contains about 8 PPM of dissolved
oxygen at room temp.
As the water is heated, the dissolved oxygen is set free .
Dissolved oxygen reacts with the iron of boiler in
presence of water and under prevailing high temperature
to form ferric oxide (rust).
4Fe + 2H2O+ O2 4Fe(OH)2
4Fe(OH)2 + O2 2 [Fe2O3 . 2H2O]
Ferric Oxide (Rust)
Corrosion in boilers is due to the following reasons:-](https://image.slidesharecdn.com/unit2boilerproblemandsofteningmethods1681804779-230604120345-d04e8813/85/unit-2_Boiler-Problem-and-Softening-methods_1681804779-pptx-25-320.jpg)










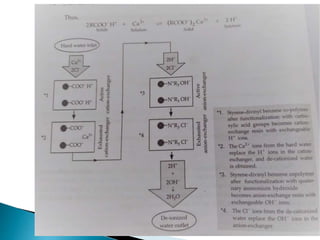
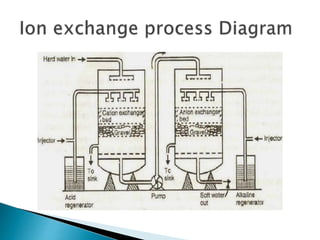
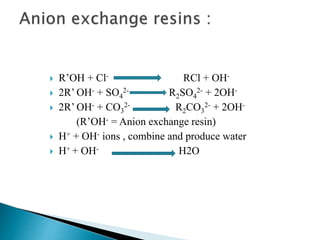
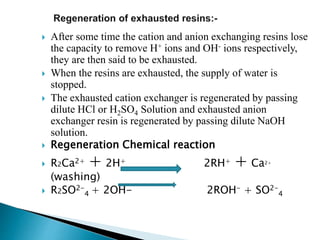


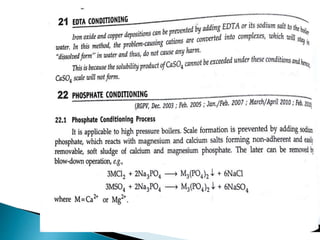
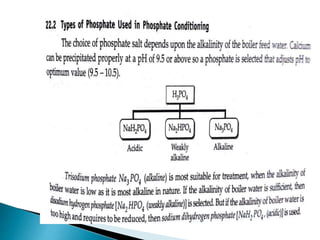

![ Carbonate Conditioning: In low pressure boilers, scale
formation can be avoided by adding sodium carbonate
to boiler water when CaSO4 is converted into calcium
carbonate in equilibrium
CaSO4 + Na2CO3 CaCO3 + Na2SO4
(iv) Calgon Conditioning: It involves adding calgon
(NaPO3)6 to boiler water. It prevents the scale & sludge
formation by forming soluble complex compound with
CaSO4.
Na2[Na4(PO3)6] 2Na+ + [Na4P6O18]2-
calgon
2CaSO4 + [Na4(P6O18)]2- [Ca2P6O18]2- + 2Na2SO4
Soluble complex ion](https://image.slidesharecdn.com/unit2boilerproblemandsofteningmethods1681804779-230604120345-d04e8813/85/unit-2_Boiler-Problem-and-Softening-methods_1681804779-pptx-56-320.jpg)
