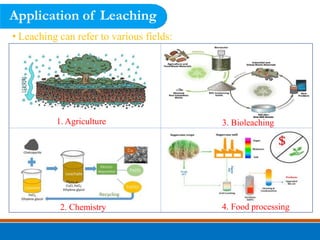
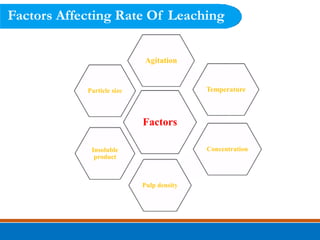
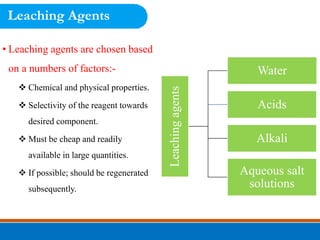
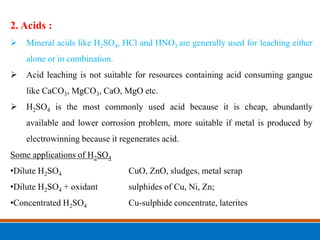
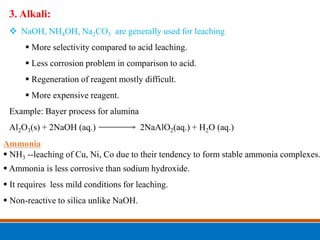
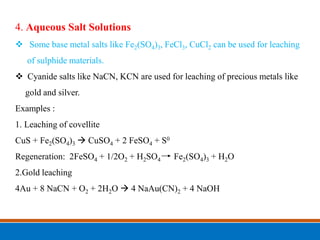
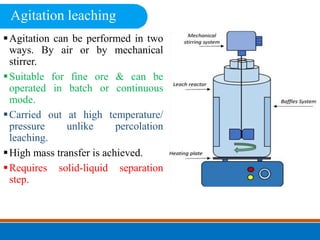
This document discusses the basics of leaching, including the principle, applications, factors affecting the rate, agents, and methods. Leaching is a solid-liquid separation process that extracts a substance from a solid material using a liquid. It occurs through contact between a solvent and solid matrix, followed by separation. Common applications include agriculture, chemistry, bioleaching and food processing. Factors like agitation, temperature, concentration and particle size influence the leaching rate. Water, acids, alkalis and salt solutions are typical leaching agents. Methods include in-situ, dump, heap, vat and agitation leaching.