The document discusses water quality and drinking water standards. It provides information on various types of water quality parameters including physical, chemical, and biological characteristics. It describes factors that affect water quality such as contamination from living organisms, solids, and dissolved materials. It also outlines primary and secondary drinking water standards from organizations like the WHO and EPA, which establish limits for parameters to protect human health and aesthetic quality. Regular water quality monitoring is emphasized to ensure standards are being met.









![Alkalinity
In addition to their mineral origin, these substances can originate from CO2 present in atmosphere and
microbial decomposition of organic materials. The reaction are
CO2 + H2O ↔ H2 CO3 (i) H2 CO3 ↔ H+ +HCO3 (ii) (bicarbonate) HCO3 ↔ H+ +CO3 (iii) (carbonate)
CO3 + H2O ↔ HCO3 + OH- (iv) (hydroxyl or hydroxide)
The relative quantities of each are a function oh pH. Hydroxyl ion concentration are significant
at pH≥ 10, the carbonate concentration below 8.3 are not significant. The bicarbonate concentration
are usually in the range of pH values of 4.5 to 8.3.
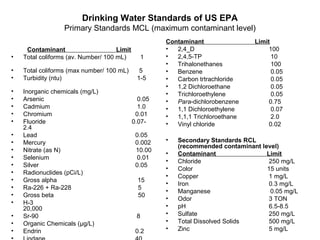
pH : pH is a measure of the free hydrogen ion (H+) concentration in water. Water and other chemicals
in solution will ionized to a greater or lesser degree. The ionization reaction of water may be written
as
HOH ↔ H+ + OH- .The concentration of H and OH can be written as [H][OH] = Kw = 10-14 at 20 C0
Taking log of both sides Log [H] + Log [OH] = -14 let ( – log )= p then pH + pOH = 14. pH= -log[H+].
In neutral condition [H] = [OH] ; hence pH = pOH = 7. Thus pH is the negative logarithm of hydrogen
ion concentration. Increasing acidity leads to higher values of [H]; thus to lower values of pH. Low pH
is associated with acidity, high pH with causticity (alkalinity). Acceptable value for drinking water is 6.5
to8.5
pH( Hydrogen ion concentration)
1----------------------------7------------------------14
Acidic Basic
• Signifance:- chemical coagulation disinfection water softening corrosion control](https://image.slidesharecdn.com/waterpropertiespressureflocculationcoagulation-141103083535-conversion-gate01/85/Water-properties-pressure_flocculation-coagulation-11-320.jpg)
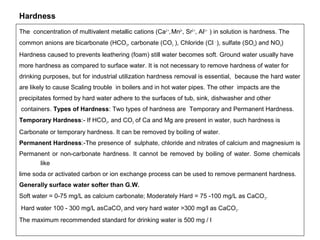
![Natural Process of Hardness
. In natural process as rain water enters the
top soil, the respiration of
microorganisms increases the CO2
content of the water. The CO2 reacts
with water to form carbonic acid
(H2CO3).The lime stone (CaCO3 and
Mg CO3) reacts with carbonic acid
forming calcium carbonate [Ca(HCO3)2]
and magnesium bicarbonate
[Mg(HCO3)2]. The Calcium and
Magnesium carbonate are insoluble in
water , while bicarbonate are soluble in
water causing hardness in water.
Similarly the Gypsum (CaSO4 ) and Mg
SO4 may also cause hardness of water
present in subsoil layers.
Rain
Top soil Bacterial Action CO2
Sub soil CO2 + H2O H2CO3
Lime stone CaCO3(s)+ H2CO3 Ca (HCO3)2
MgCO3 (s) + H2CO3 Mg (HCO3)2](https://image.slidesharecdn.com/waterpropertiespressureflocculationcoagulation-141103083535-conversion-gate01/85/Water-properties-pressure_flocculation-coagulation-13-320.jpg)