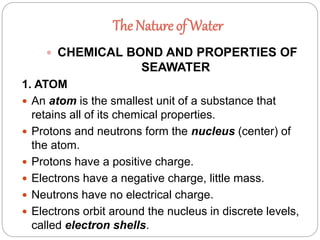
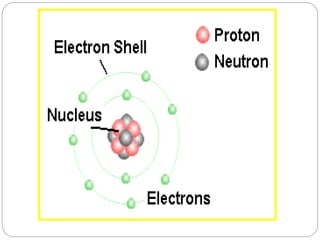
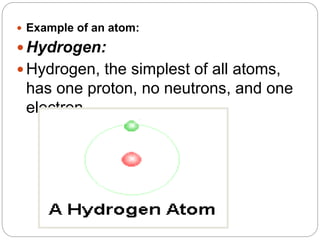
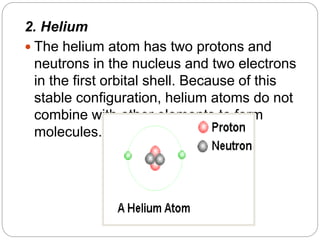
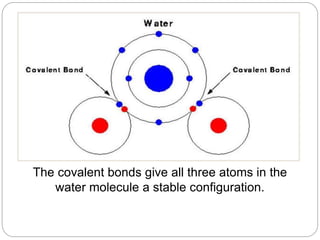
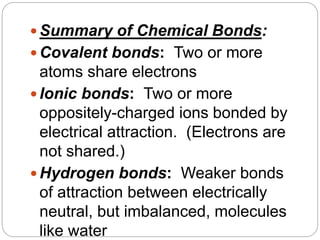
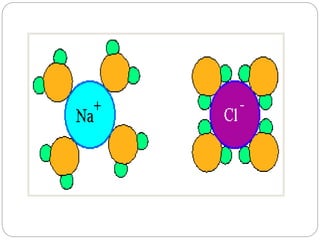
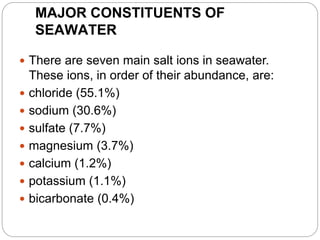
1. The document discusses the chemical and physical properties of water, including its atomic structure and bonding. Water molecules are bonded through covalent bonds and hydrogen bonding.
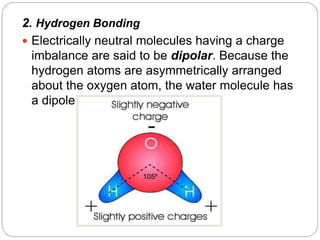
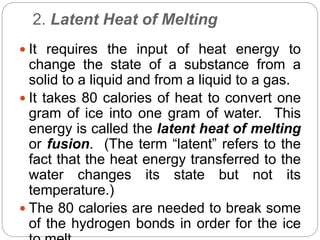
2. Hydrogen bonding gives water unique properties like high heat capacity and heat of vaporization. It takes a lot of energy to change water's state of phase.
3. Water's density peaks at 4°C and ice floats due to the structure of hydrogen bonds forming a less dense crystal structure. Hydrogen bonding also explains water's high surface tension and solvent properties.