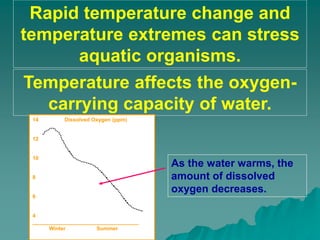
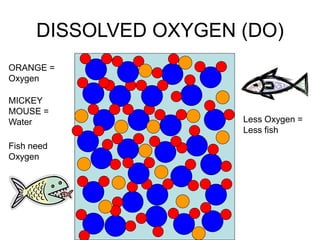
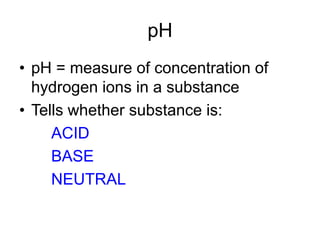
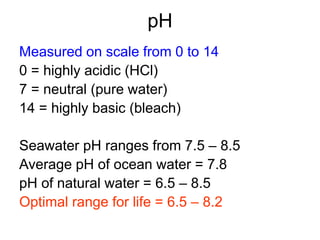
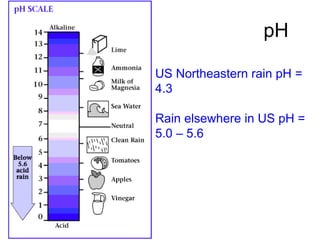
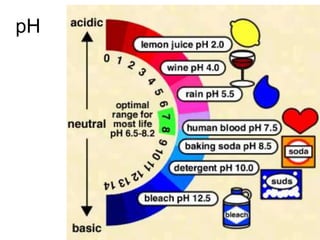
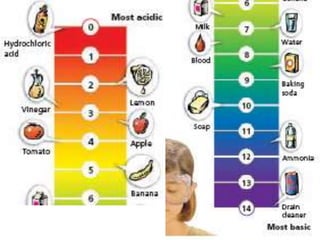
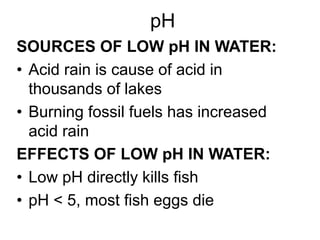
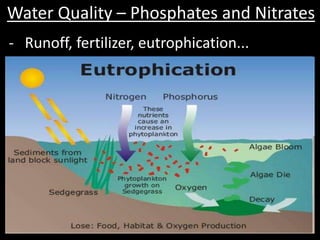
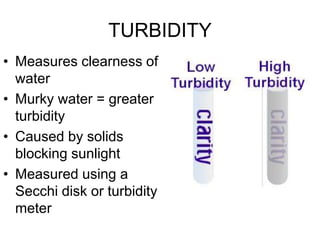
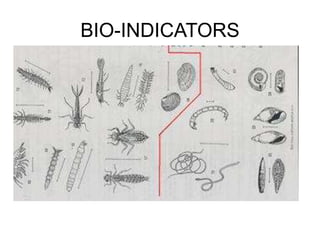
This document discusses several key factors that influence water quality: temperature, dissolved oxygen, pH, nitrogen, phosphates, turbidity, and bio-indicators. It provides details on how each factor is measured and affected by human and natural influences. For example, it explains that temperature impacts dissolved oxygen levels and organism stress, while excess nitrogen and phosphates can cause eutrophication and lower dissolved oxygen. Turbidity measures water clarity and is increased by sediments, and bio-indicators can help assess water health through presence of different organisms.