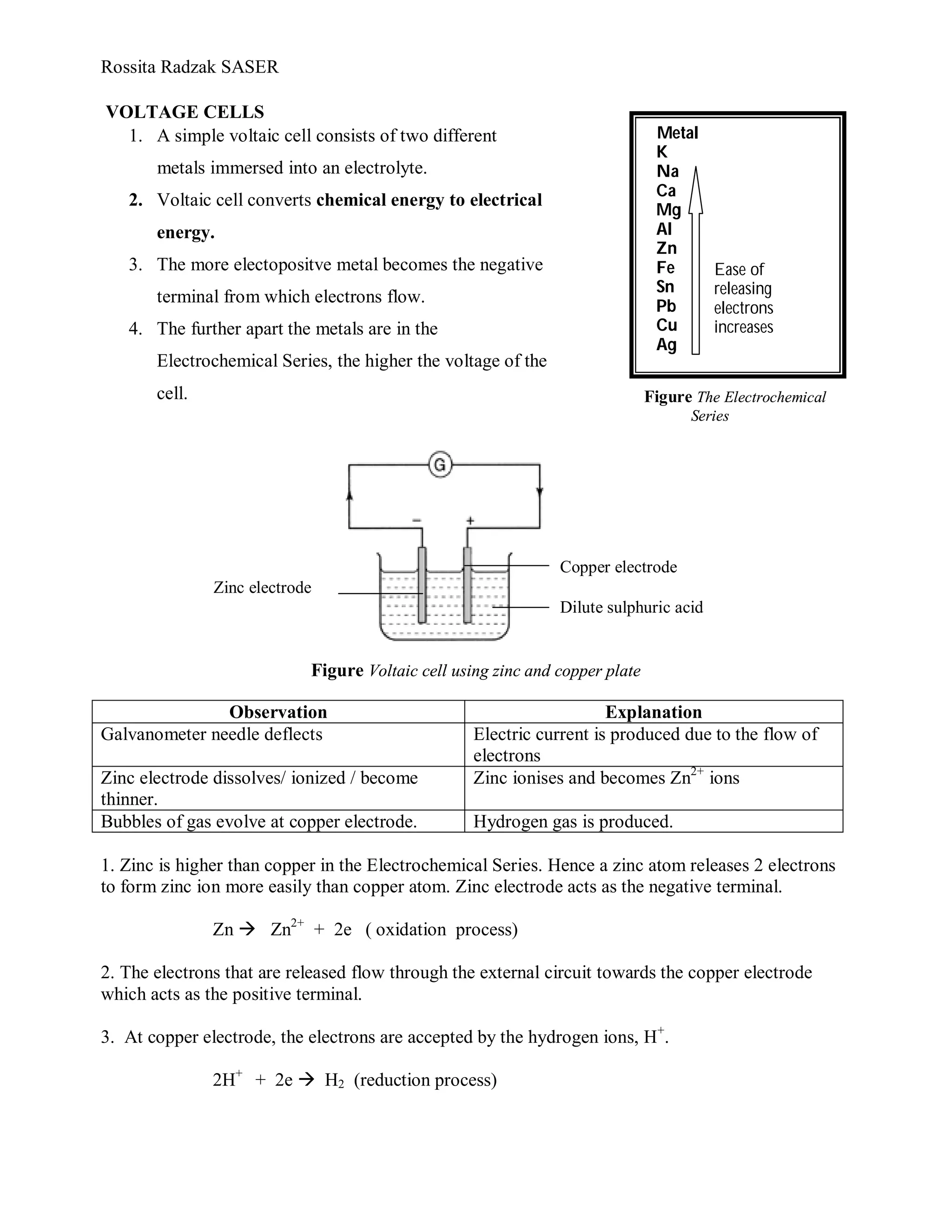
A voltaic cell converts chemical energy to electrical energy using two different metals immersed in an electrolyte. The more electropositive metal becomes the negative terminal, releasing electrons that flow through an external circuit to the other metal, which acts as the positive terminal. For example, a zinc-copper voltaic cell uses zinc and copper plates in a dilute sulfuric acid electrolyte, with zinc dissolving as ions and electrons flowing to the copper where they combine with hydrogen ions to produce bubbles of hydrogen gas. Zinc is higher than copper in the electrochemical series, so it more easily releases electrons to power the flow of current.