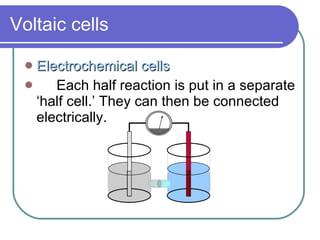
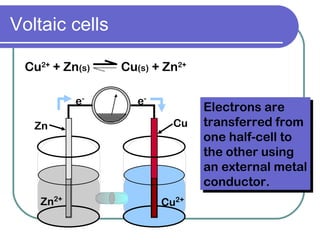
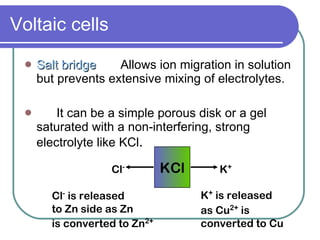
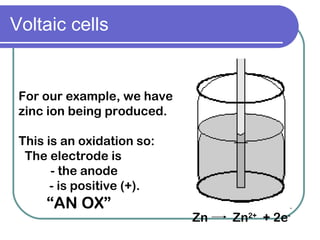
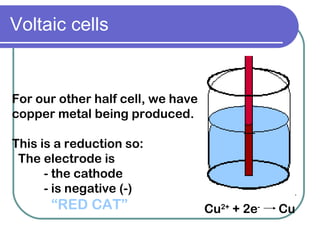
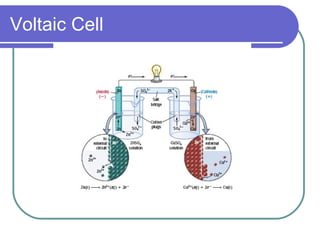
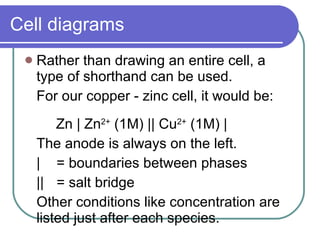
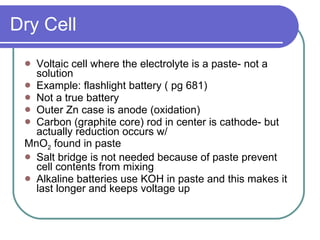
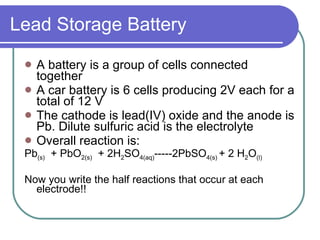
This document discusses different types of electrochemical cells including galvanic/voltaic cells which produce electrical energy from spontaneous reactions and electrolytic cells which require electrical energy for non-spontaneous reactions. It describes how voltaic cells work by separating the oxidation and reduction half reactions into two half cells connected by a salt bridge or porous disk to allow ion flow. Common examples discussed include copper-zinc voltaic cells, dry cells like flashlights, lead storage batteries in cars, and hydrogen-oxygen fuel cells.