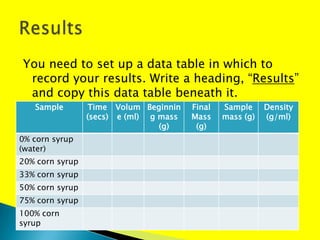
If the concentration of a corn syrup solution increases, then the density of the solution will increase and the viscosity of the solution will increase. The document provides instructions for an experiment to measure how the density and flow time (viscosity) of corn syrup solutions change with increasing concentration. Students are asked to make observations and conclusions about the relationships between concentration, density, and viscosity based on their experimental results.