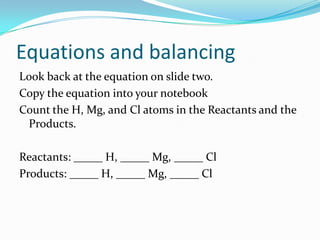
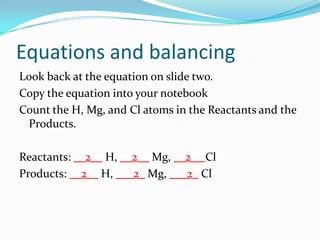
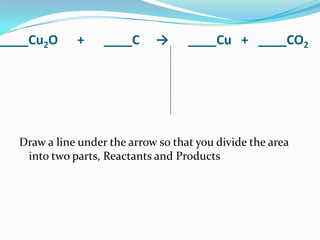
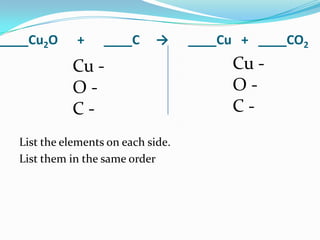
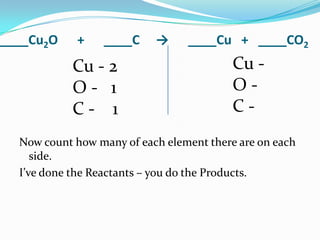
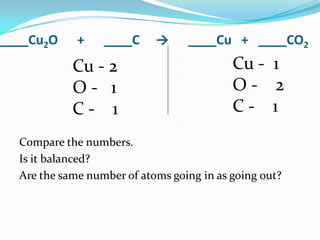
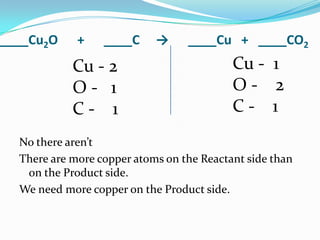
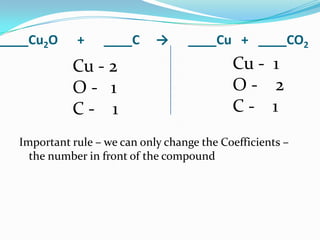
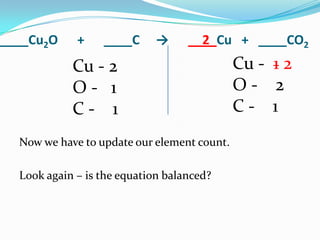
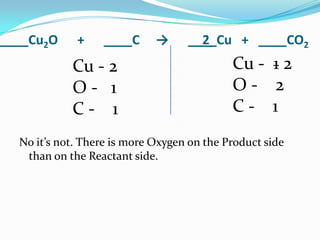
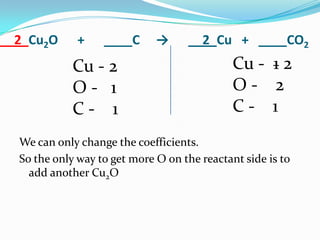
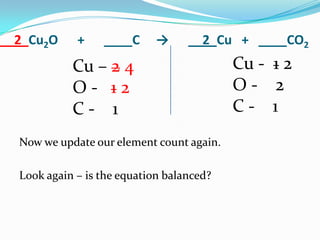
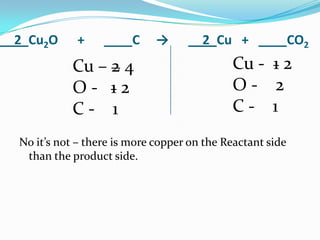
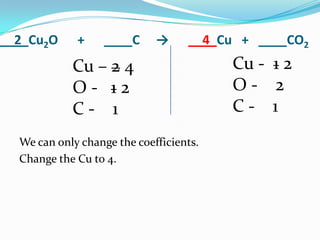
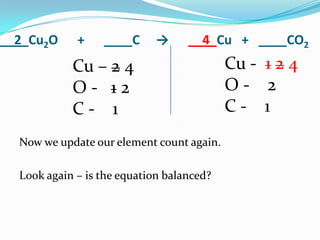
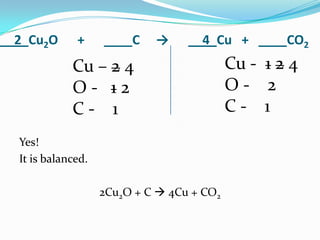
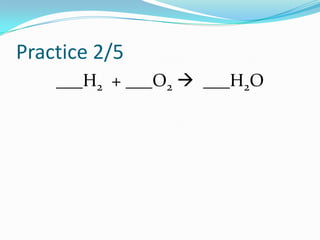
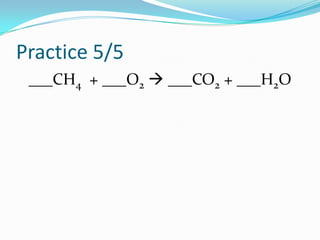
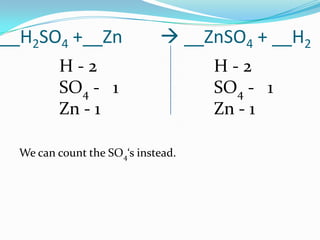
This document provides instructions for balancing chemical equations using the law of conservation of mass. It explains that matter is neither created nor destroyed in chemical reactions, it just changes form. Students are taught to write balanced chemical equations showing the same number and type of atoms on both sides of the reaction. Through an example, it demonstrates how to balance equations by adjusting coefficients in front of compounds to match the number of each element in the reactants and products.