The document discusses how the Internet of Everything (IoE) could revolutionize clinical trials through connecting patients, data, and devices. Key points:
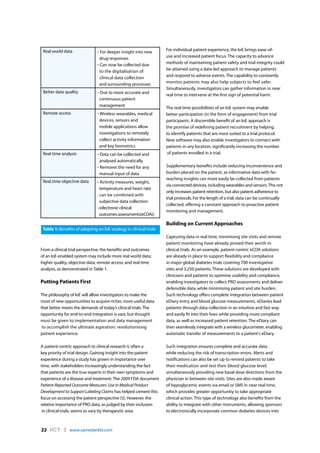
1) The IoE is being widely adopted across industries and could significantly benefit healthcare by applying it to clinical trials. This would allow continuous monitoring of patient data through connected devices.
2) An IoE system could remotely collect data from wearable devices and sensors, transmit it to servers for analysis, and enable real-time reporting. This offers benefits like instant access to risk factors and improved patient compliance monitoring.
3) While more data collection allows greater insights, challenges include evaluating large data volumes, ensuring privacy and security, and determining the best analysis protocols. Overall