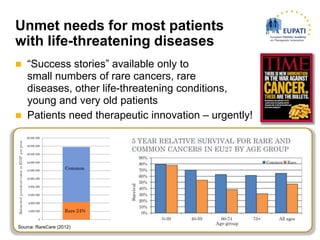
This document discusses the need for a paradigm shift in public understanding of medical research and development (R&D) to better meet patient needs. It notes that current "success stories" only help small numbers of patients, and that patients urgently need therapeutic innovation. It argues that innovation in areas like cancer, rare diseases, clinical trial design, and personalized medicine requires new approaches in R&D. It also emphasizes the important role patient organizations can play by providing insights into patient experiences and priorities to help drive research. Finally, it introduces the European Patients' Academy as an initiative aiming to train patient advocates and foster collaborative efforts to make drug development more effective.