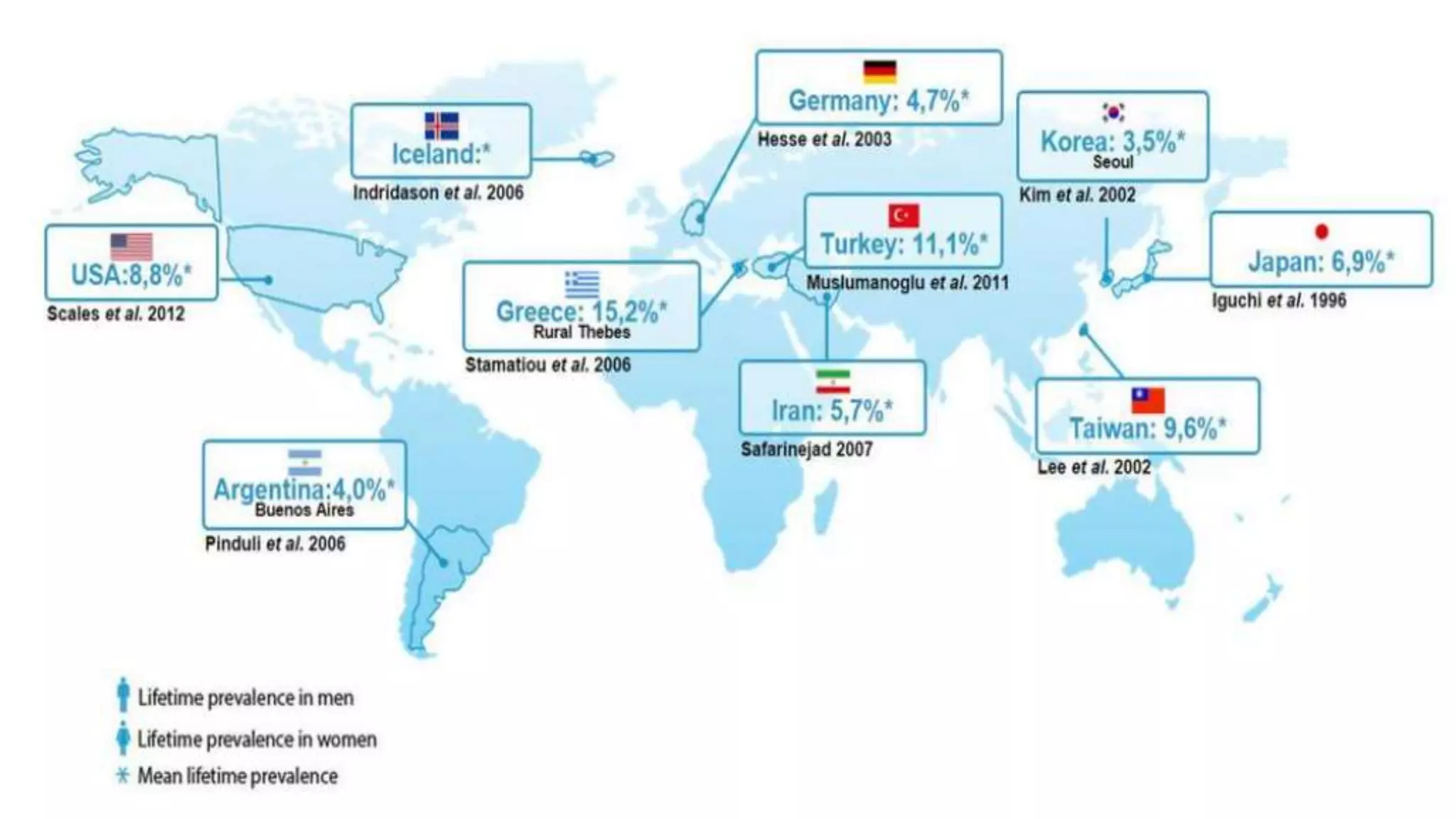
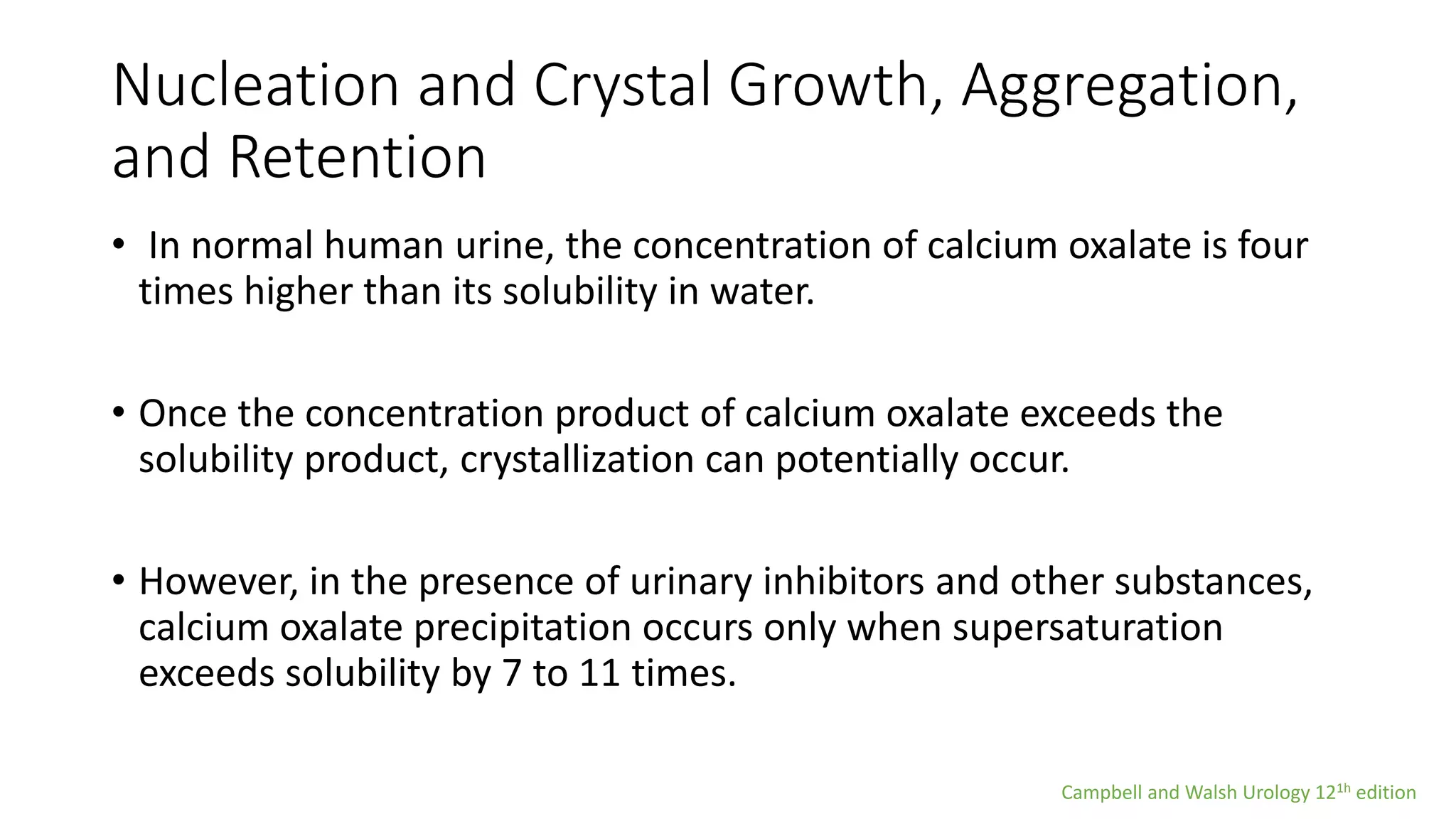
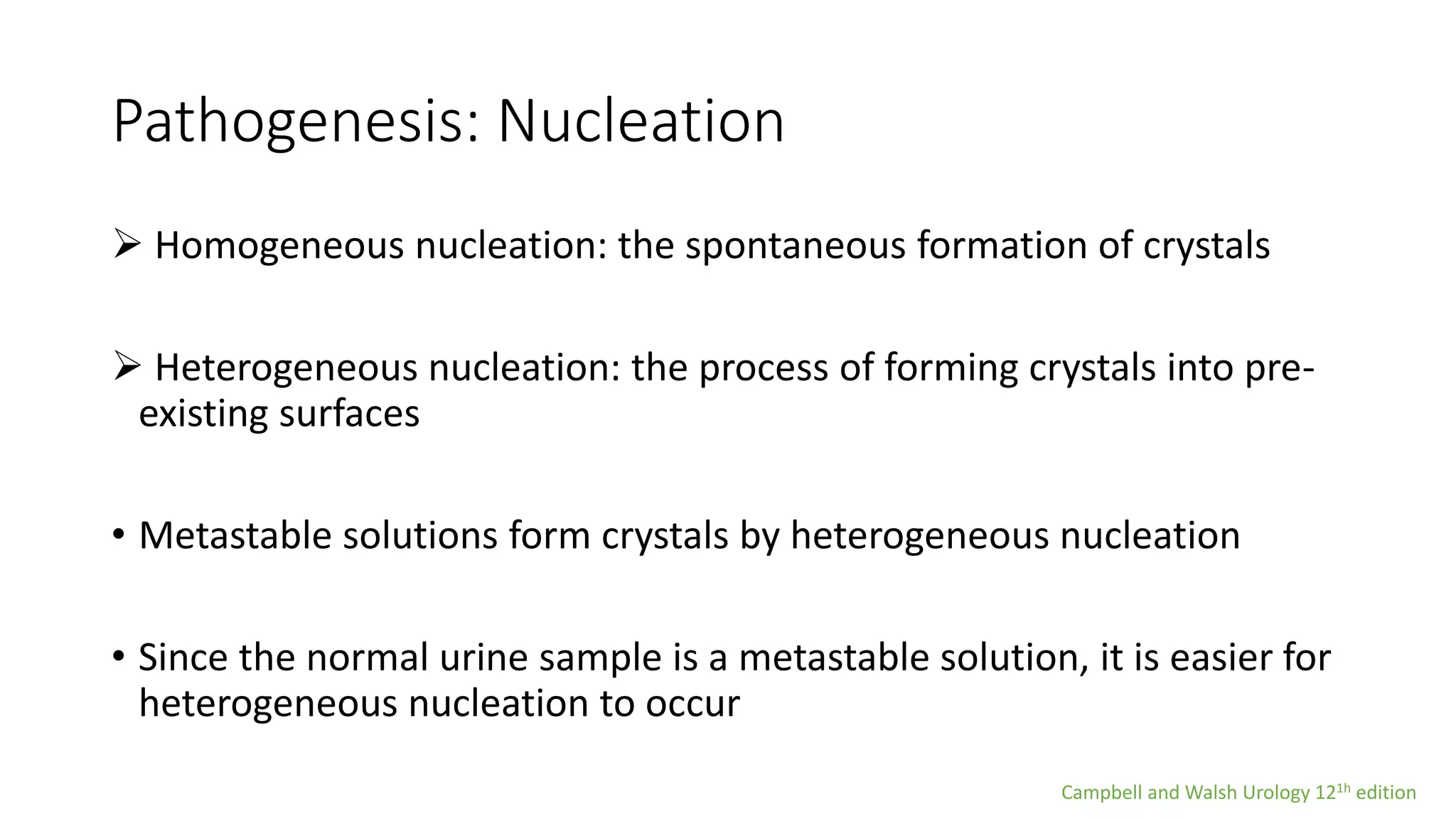
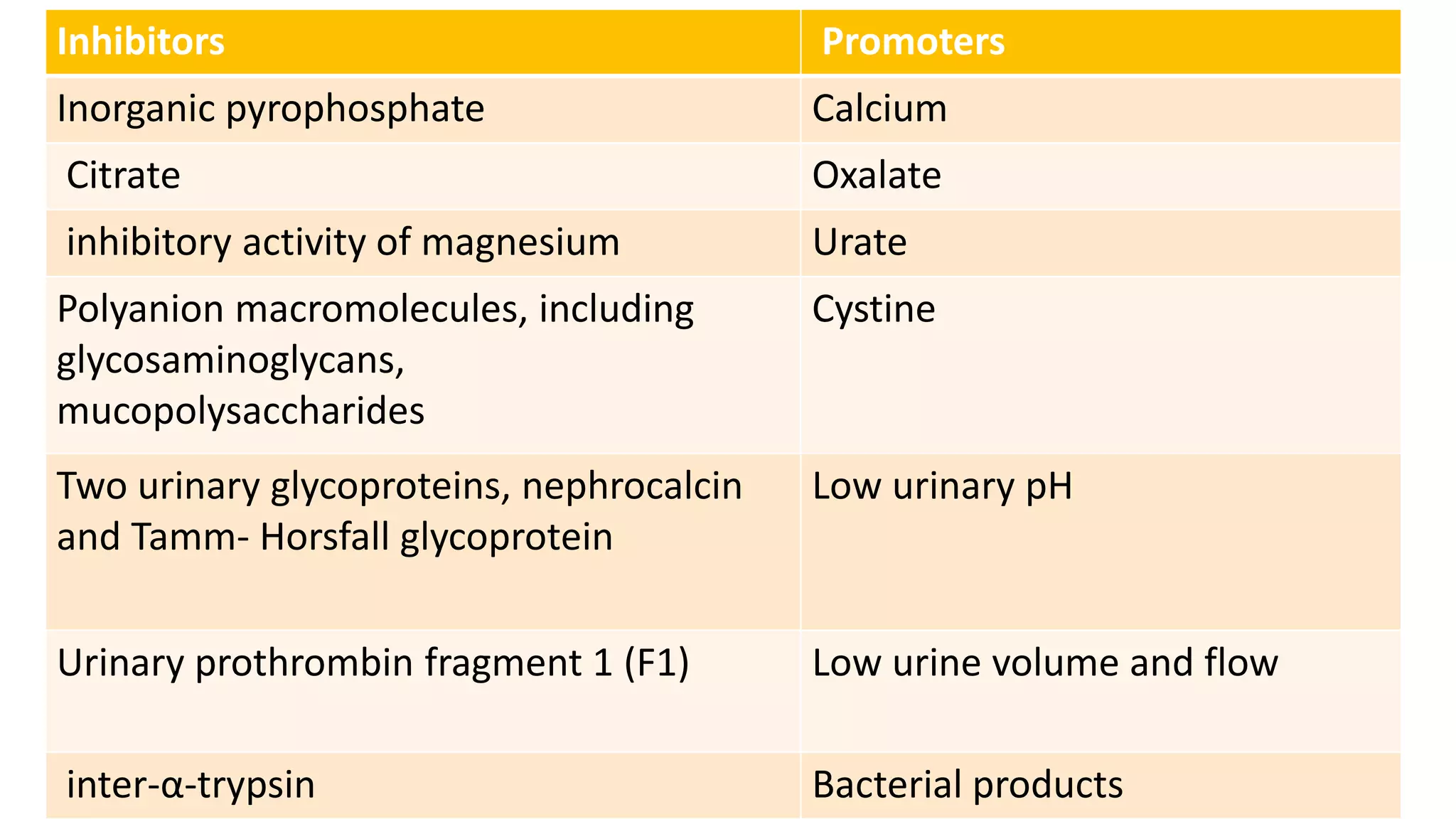
Urolithiasis refers to kidney stone formation. The lifetime prevalence of kidney stones is 1-15%, with peak prevalence occurring in adults aged 60-69 years old. Males are more affected than females. Risk factors include age, gender, geography, climate, occupation, obesity, and medical conditions like diabetes. Stone formation occurs through a process where urine becomes supersaturated, crystals nucleate, aggregate, and are retained in the kidneys. The balance between substances that promote or inhibit crystallization and stone growth determine whether stones will form.