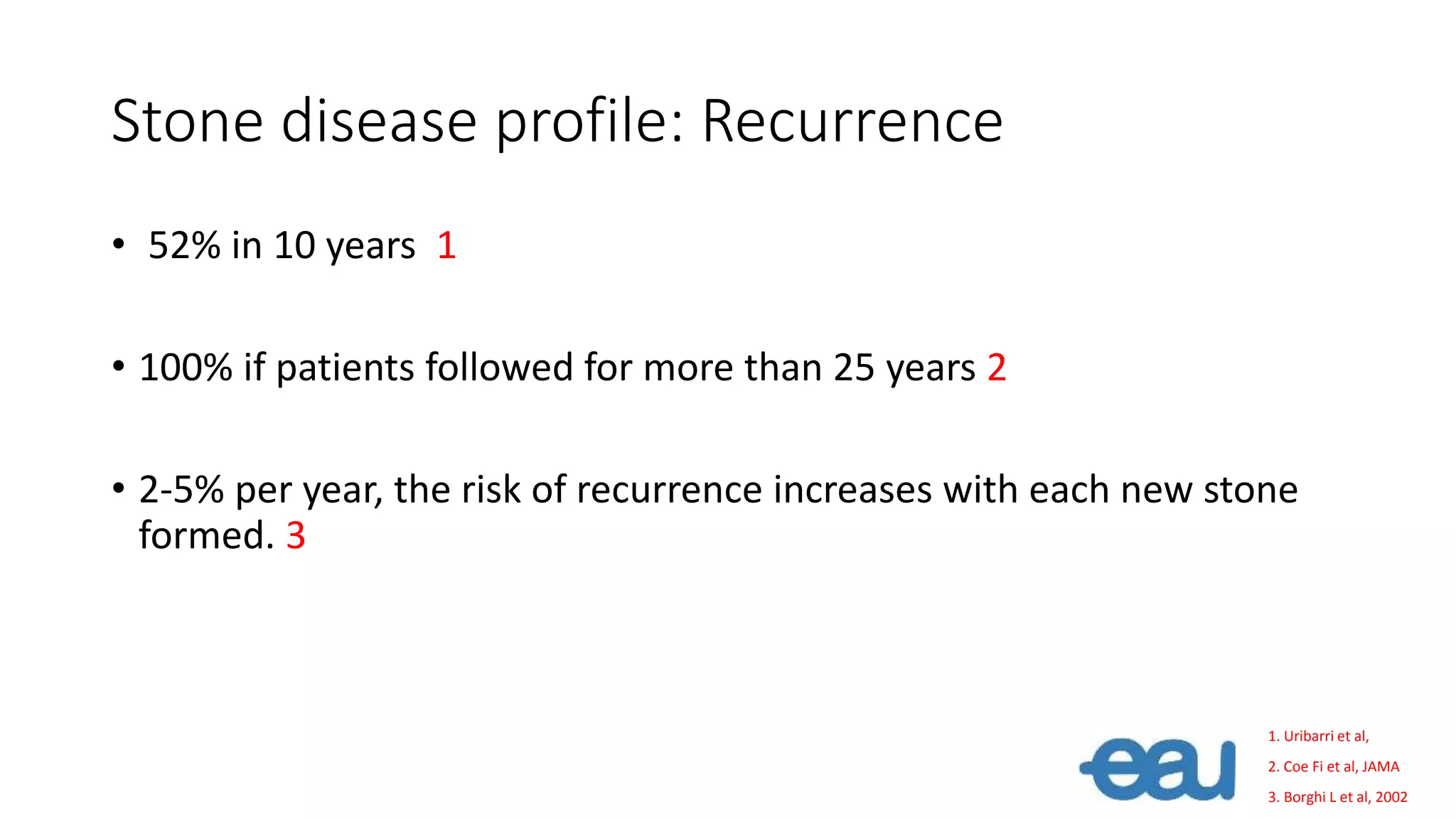
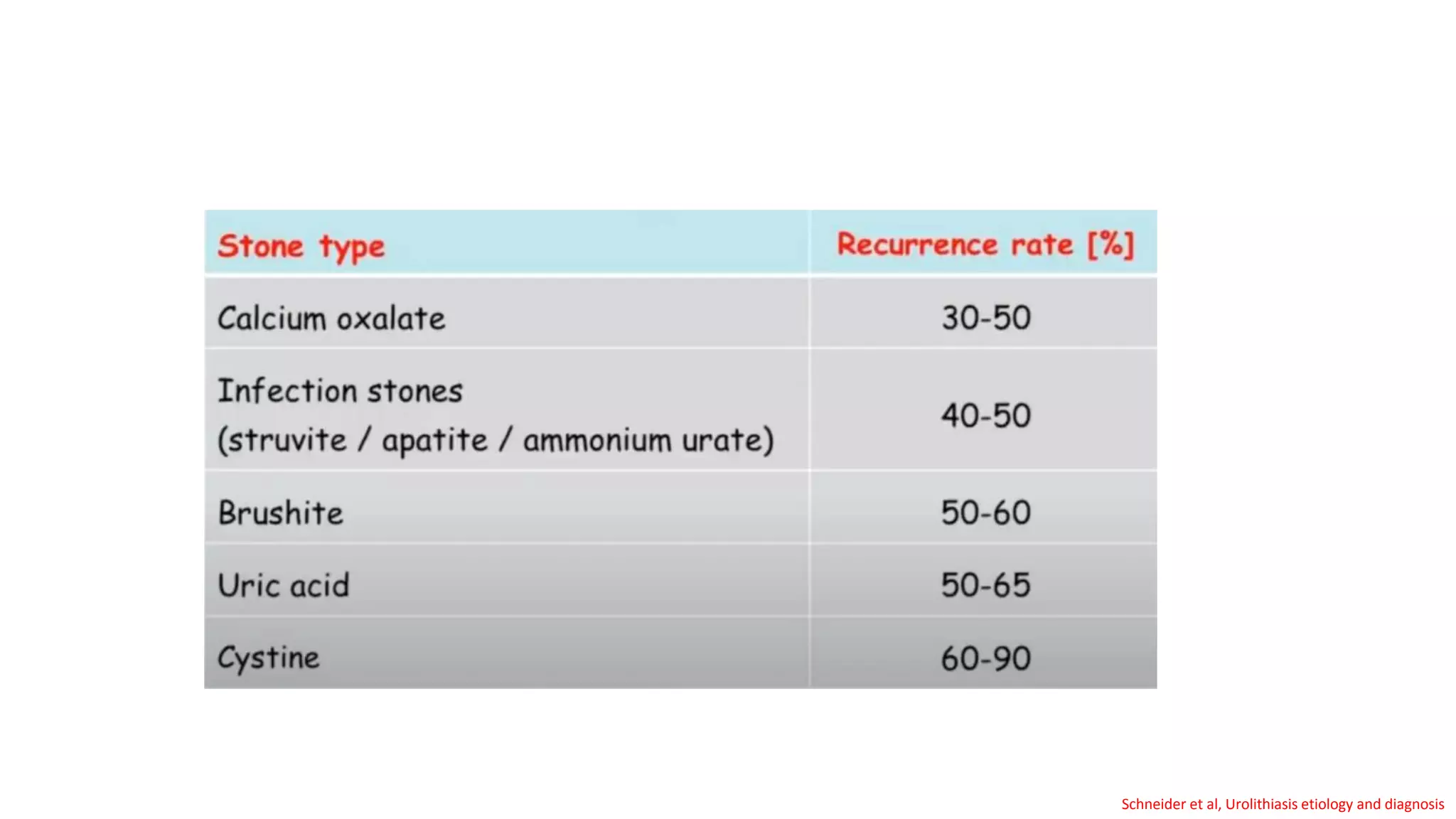
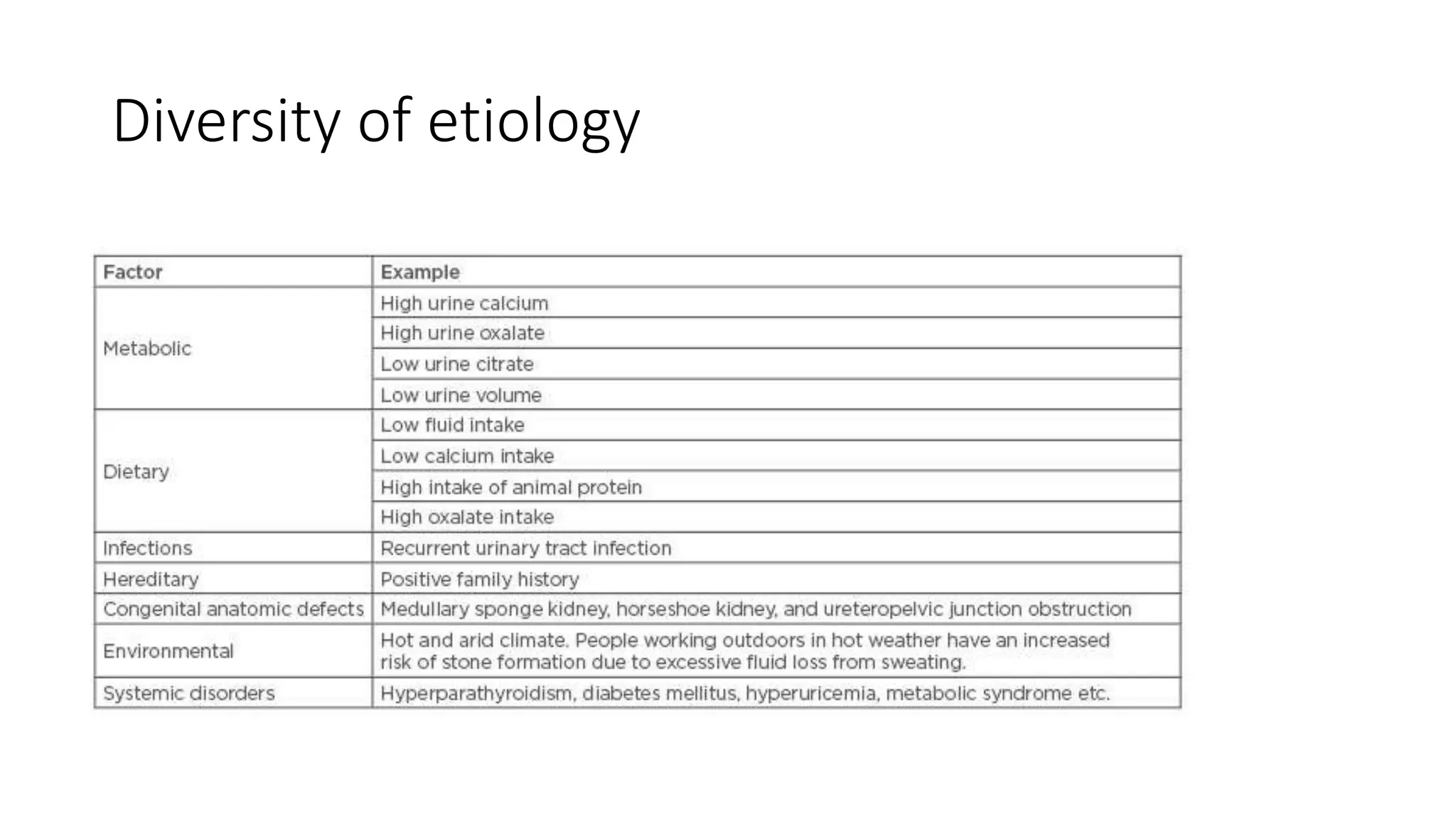
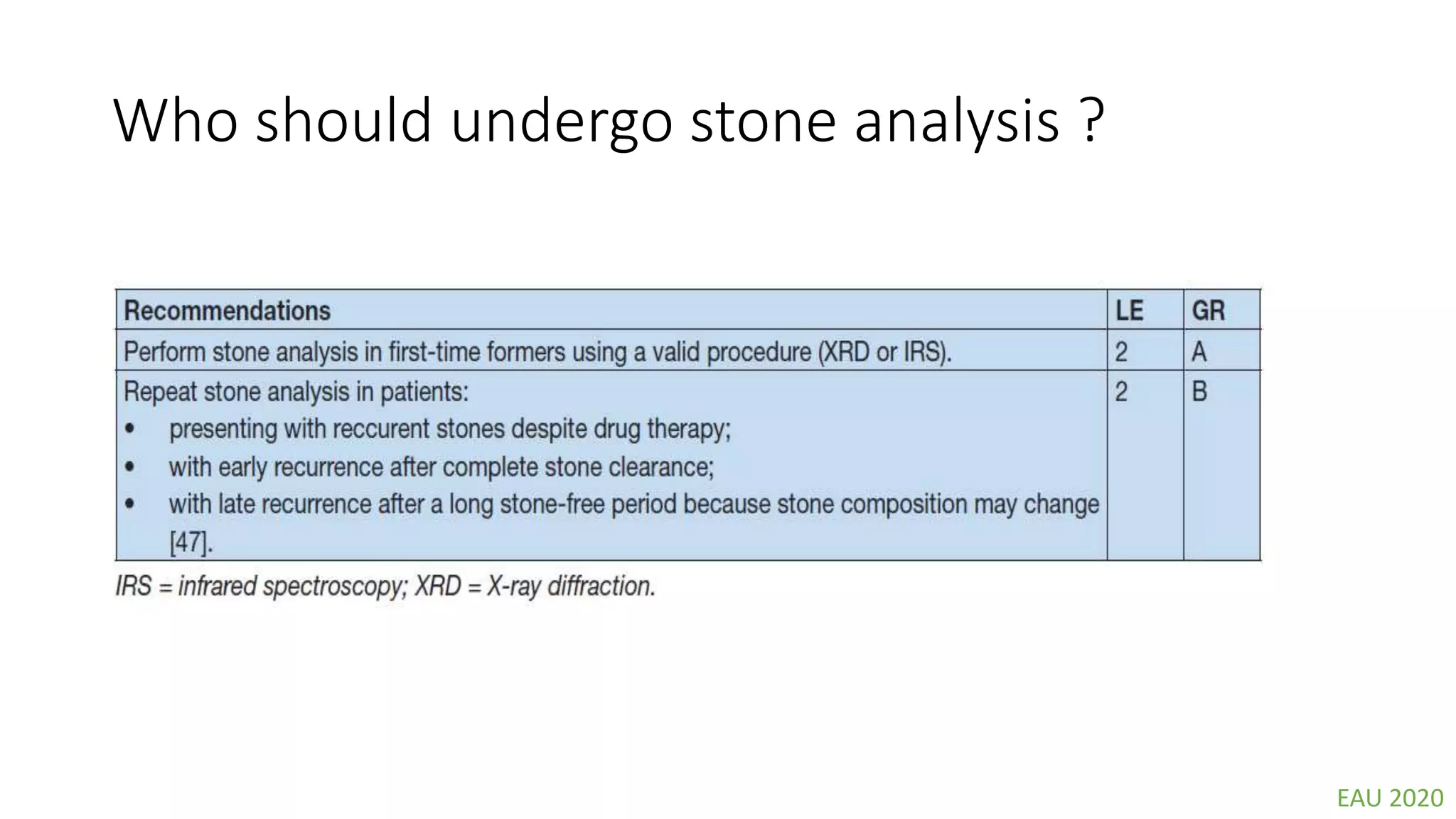
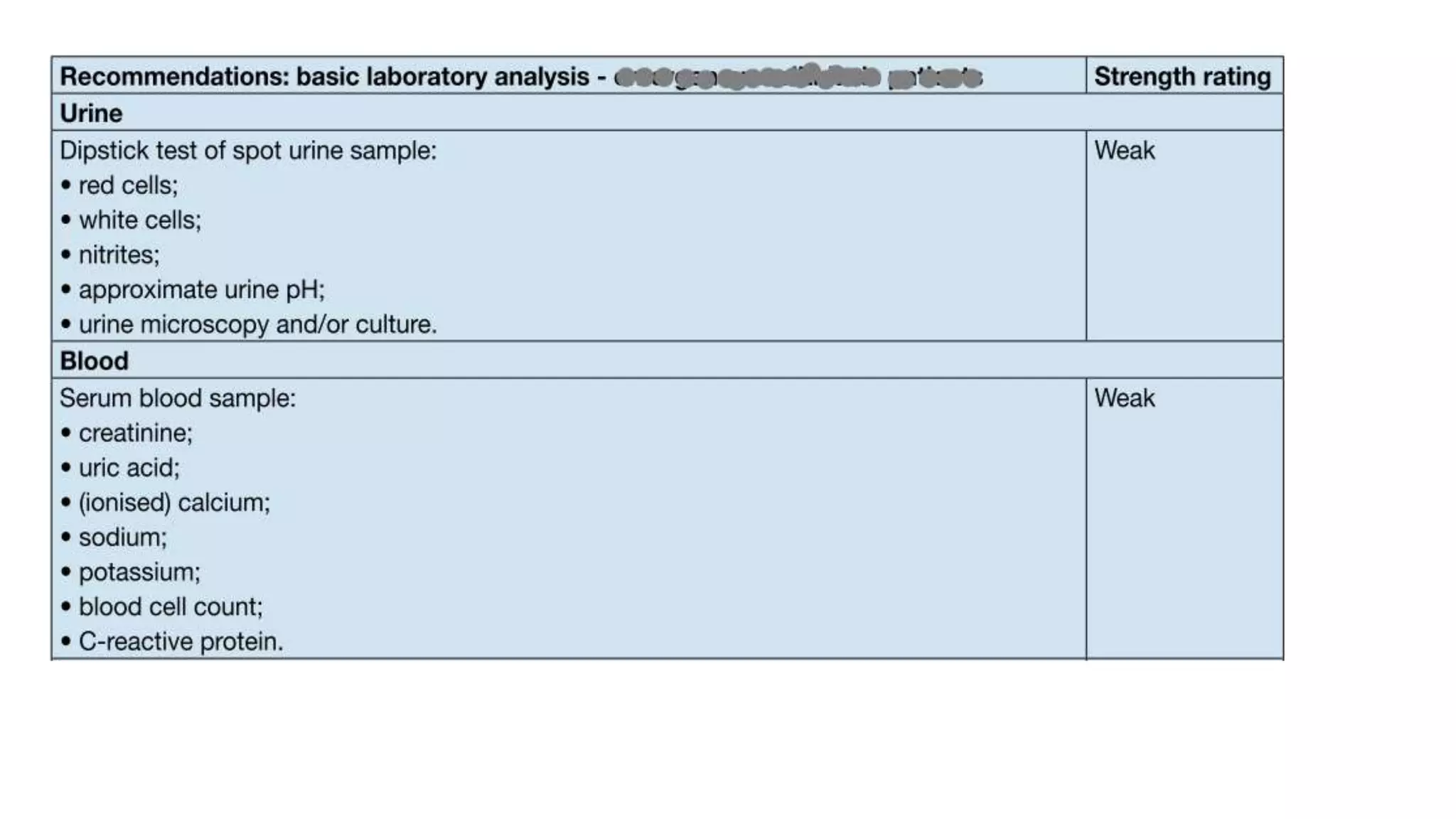
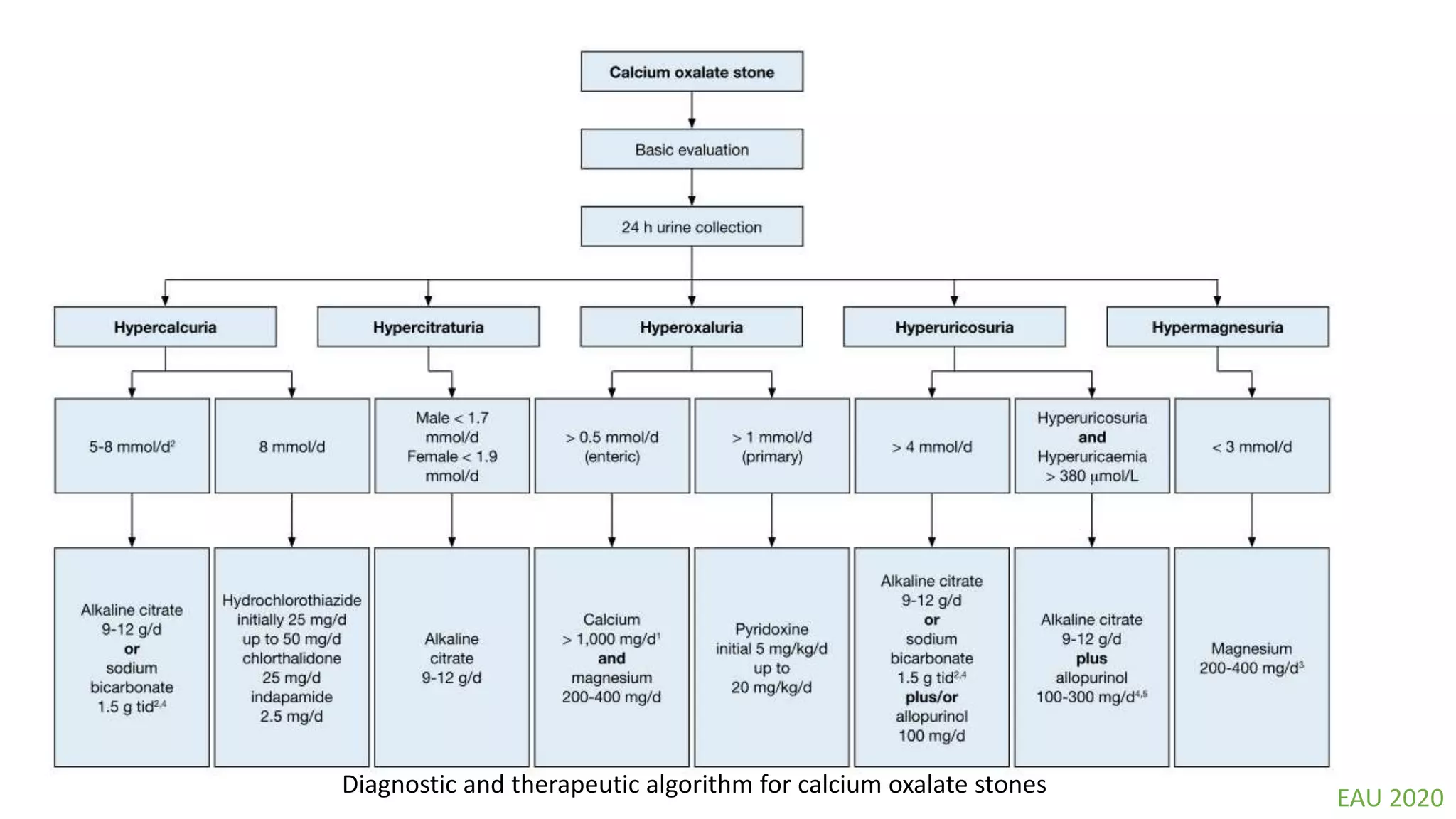
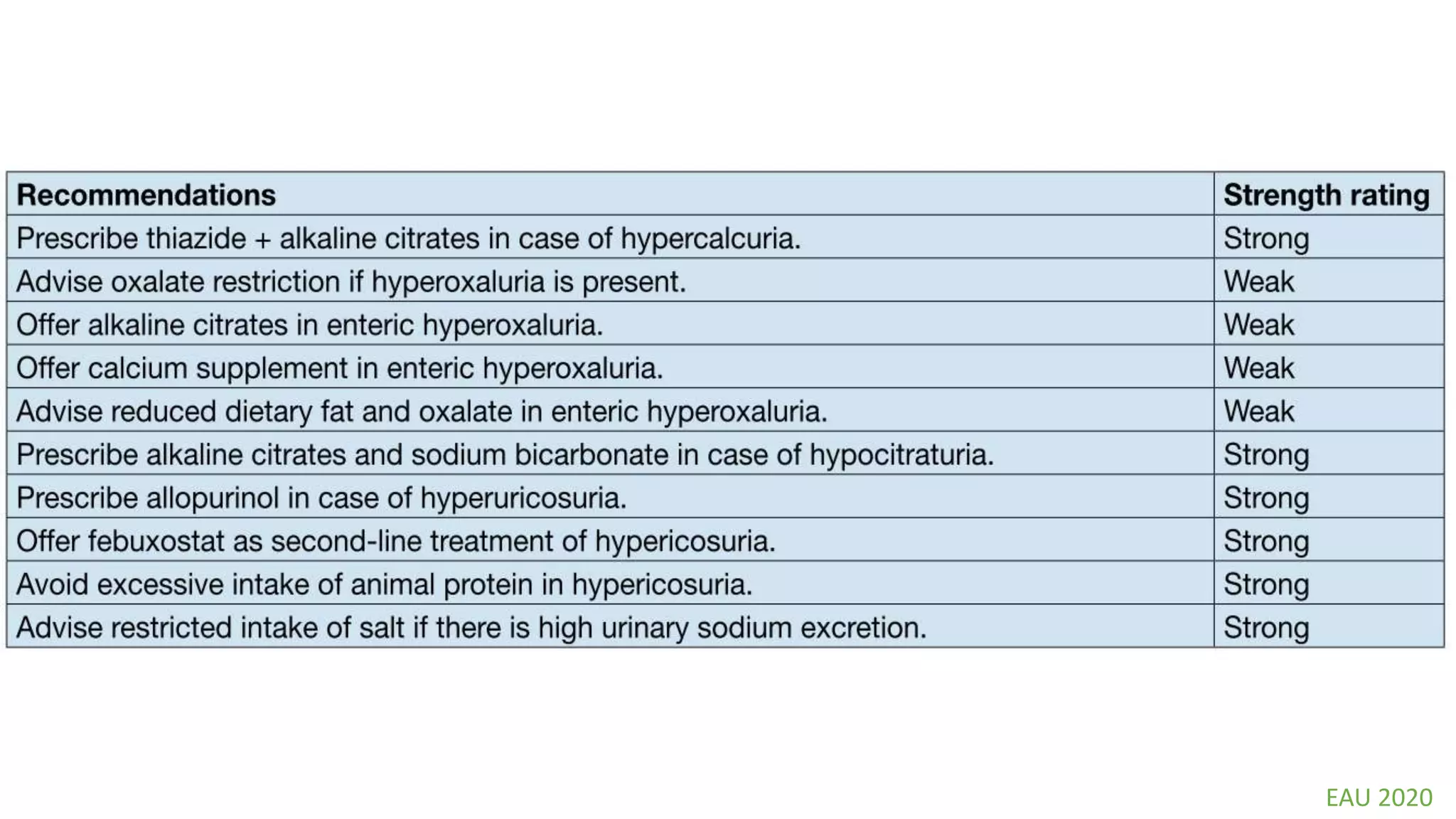
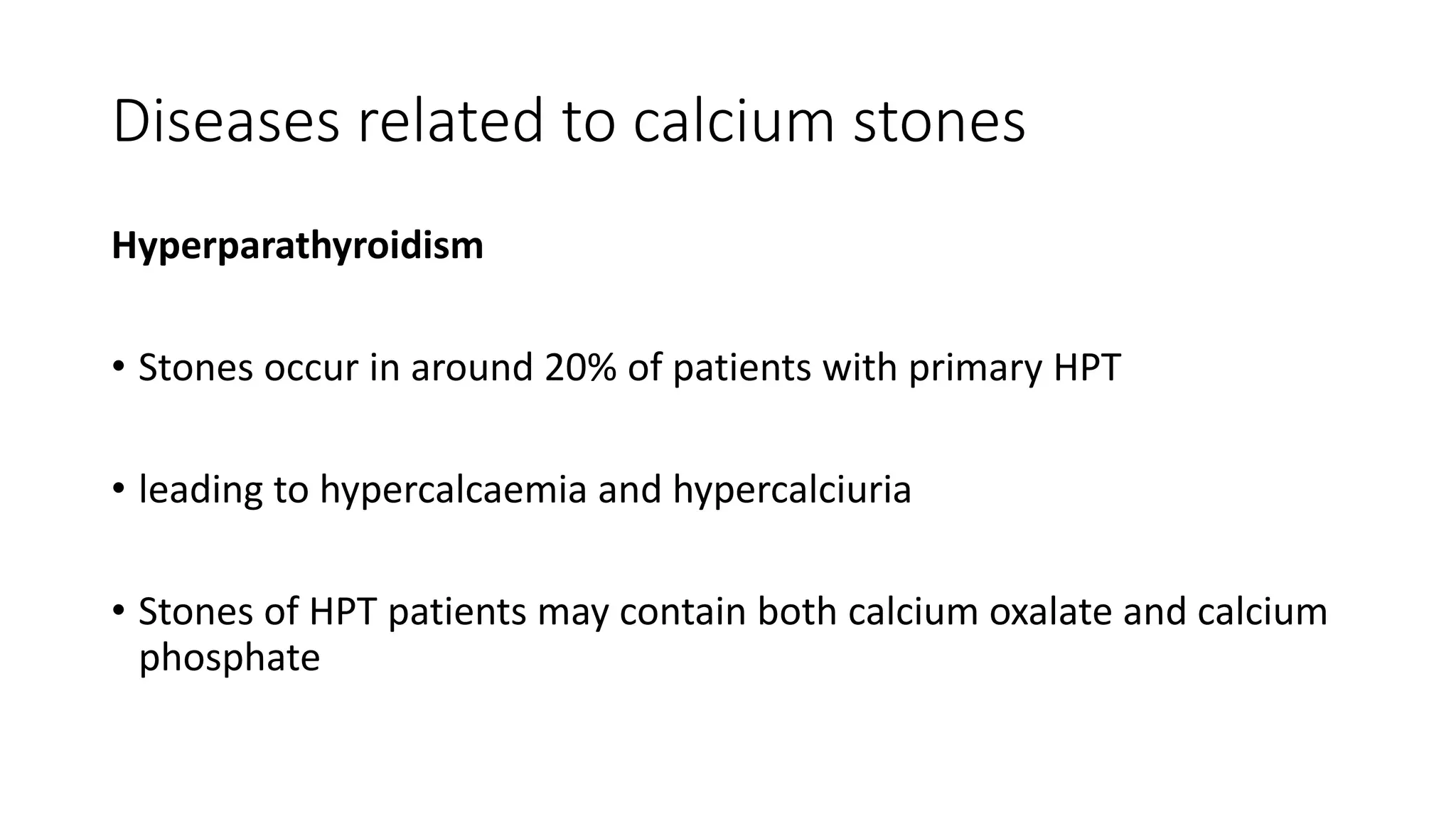
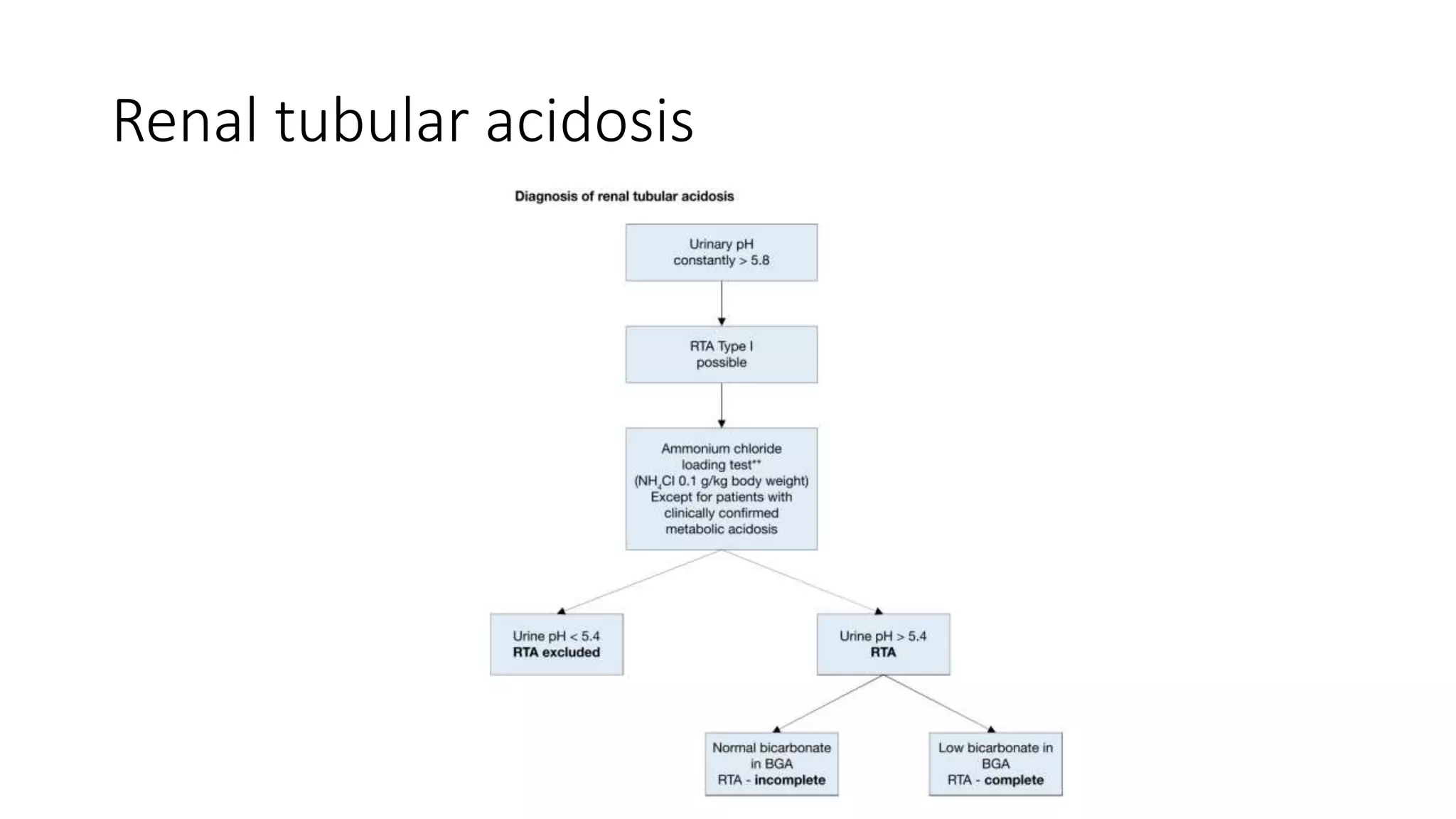
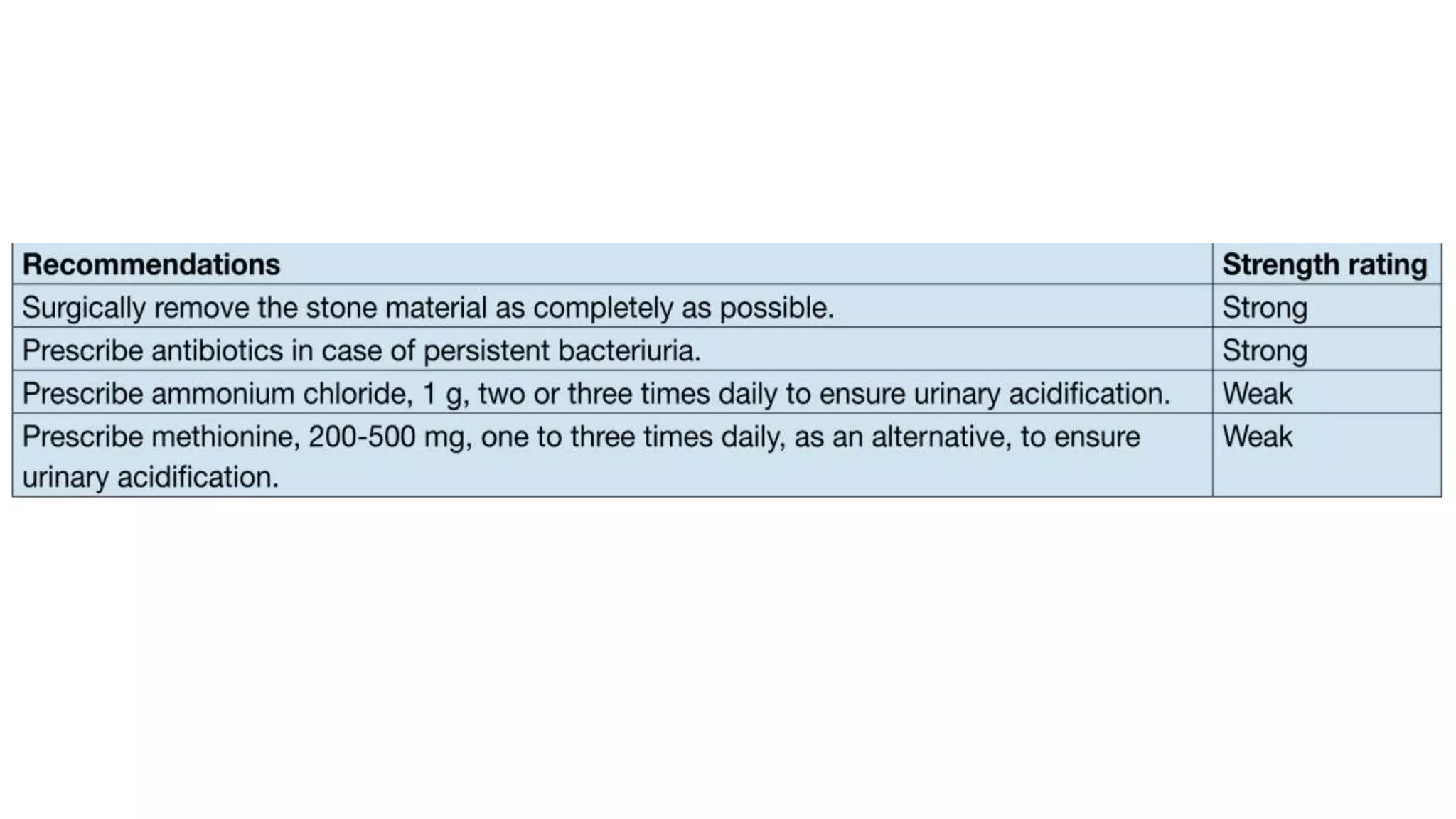
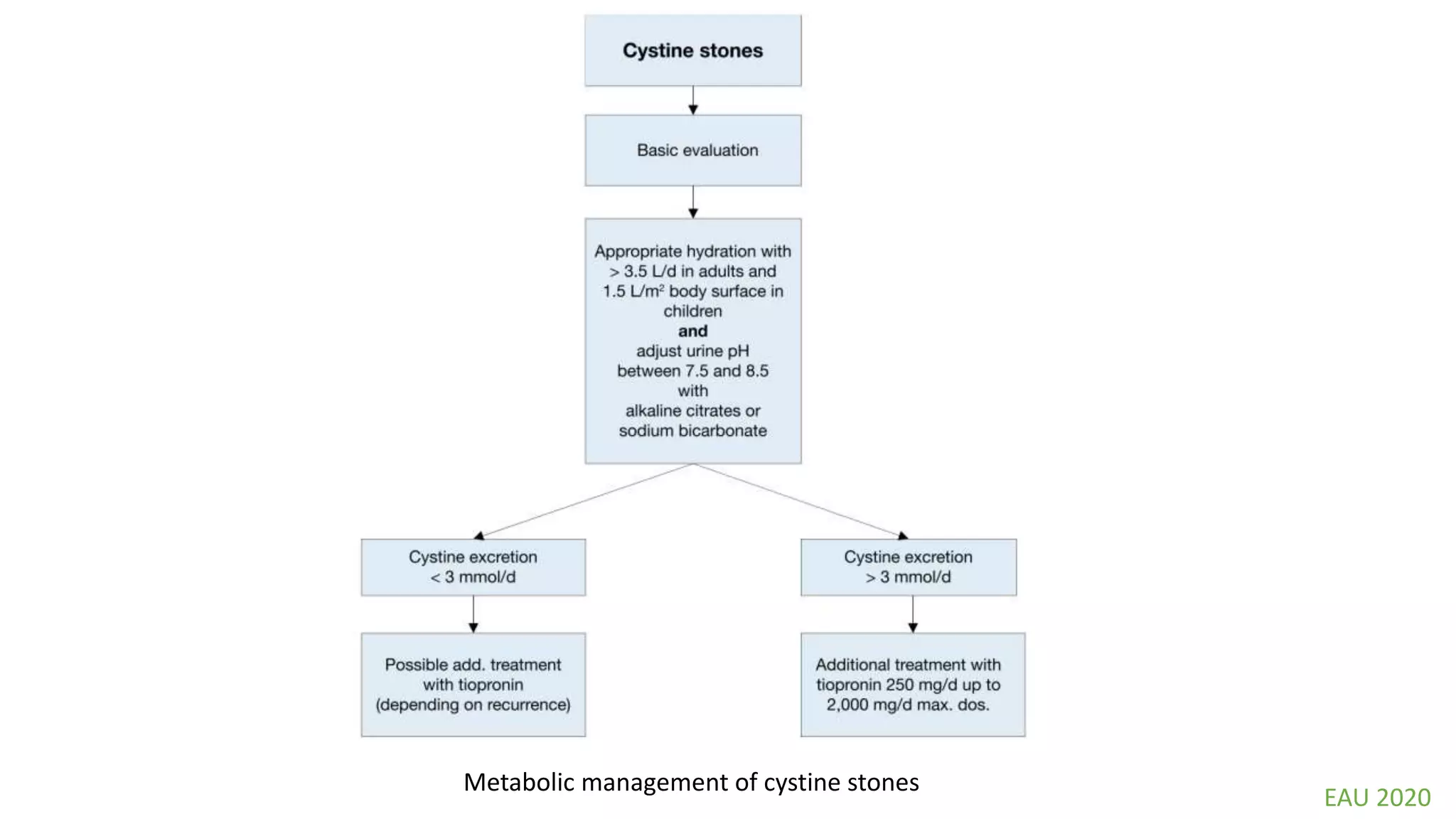
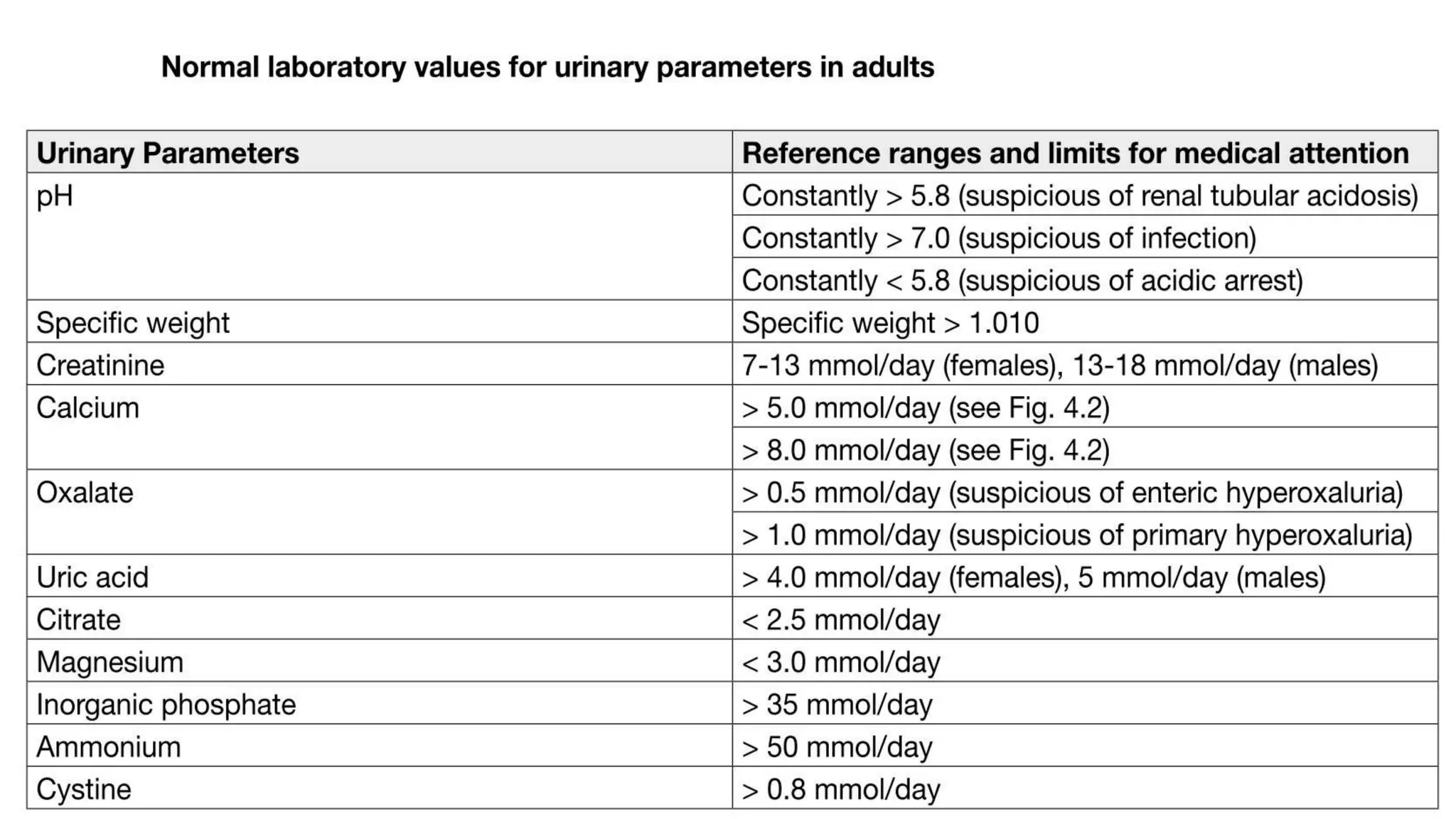
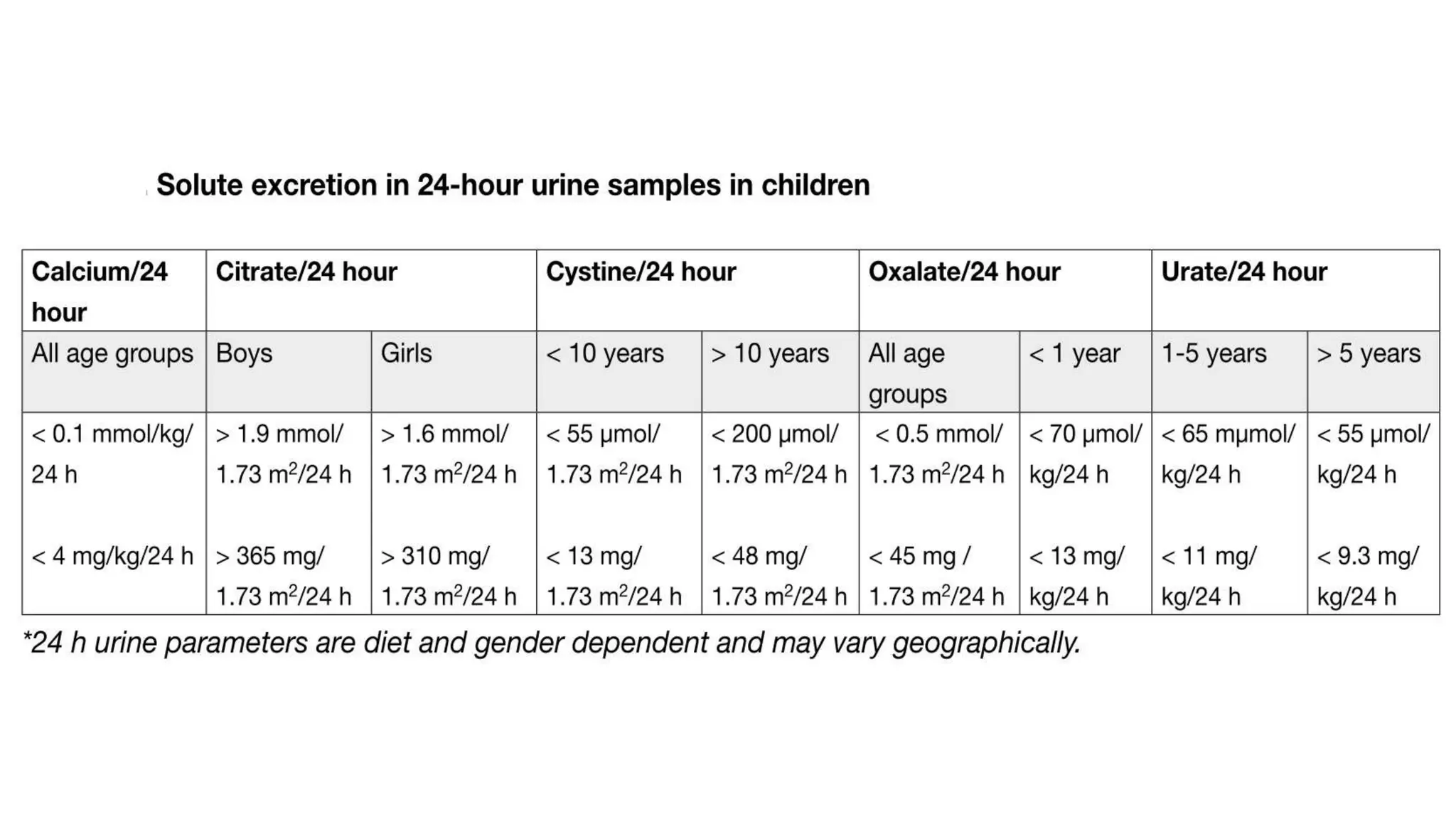
This document discusses metabolic evaluation and prevention strategies for kidney stone disease. It recommends stone analysis for all patients to classify them as high or low risk for recurrence. For high risk patients, specific metabolic evaluation includes measuring stone-related substances like calcium, oxalate, and citrate in 24-hour urine samples. Based on stone composition and test results, treatment targets the underlying metabolic abnormality to reduce recurrence rates by up to 46%. Proper stone analysis, metabolic workup, and preventive measures can minimize stone formation and risk of chronic kidney disease.