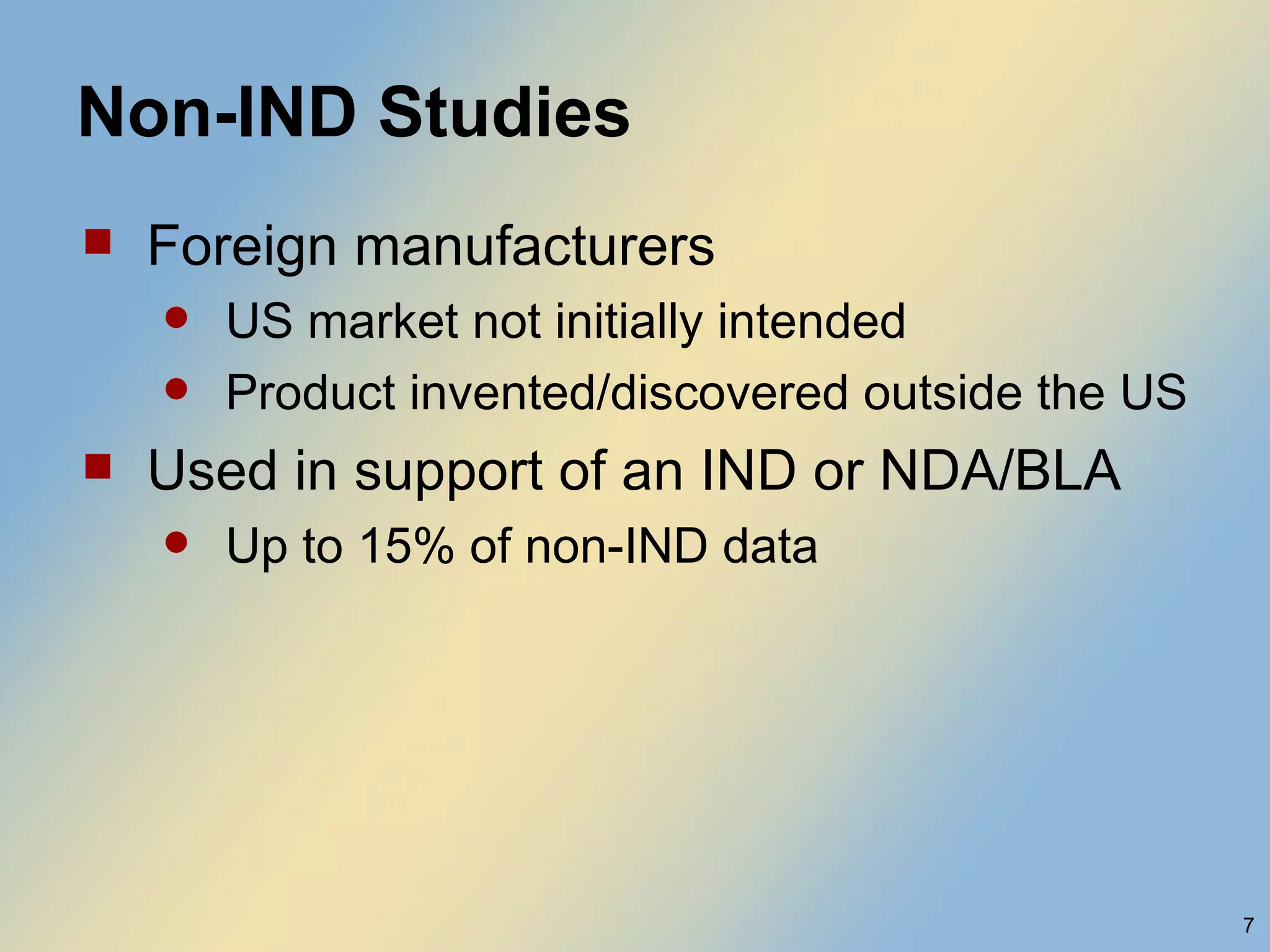
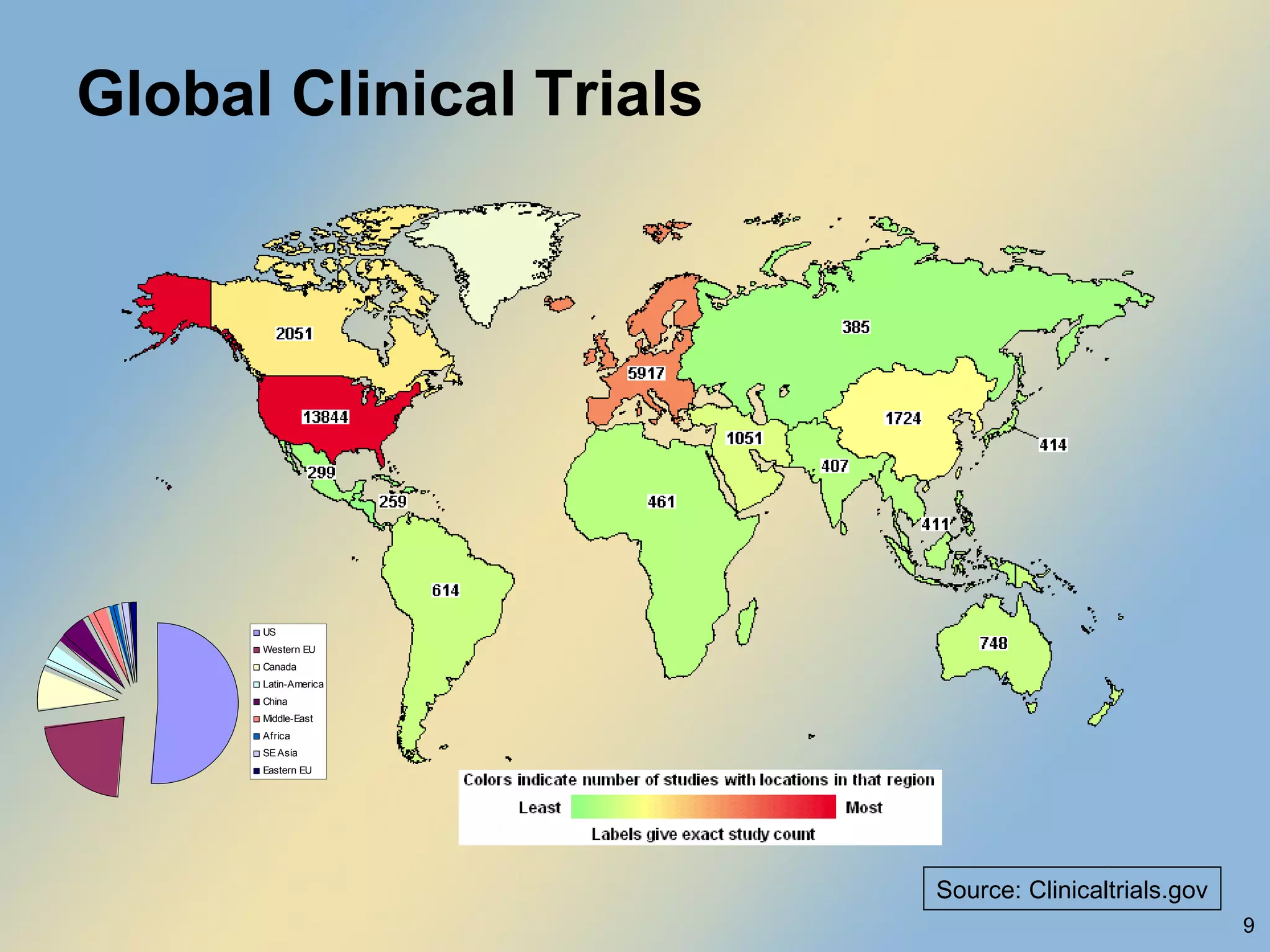
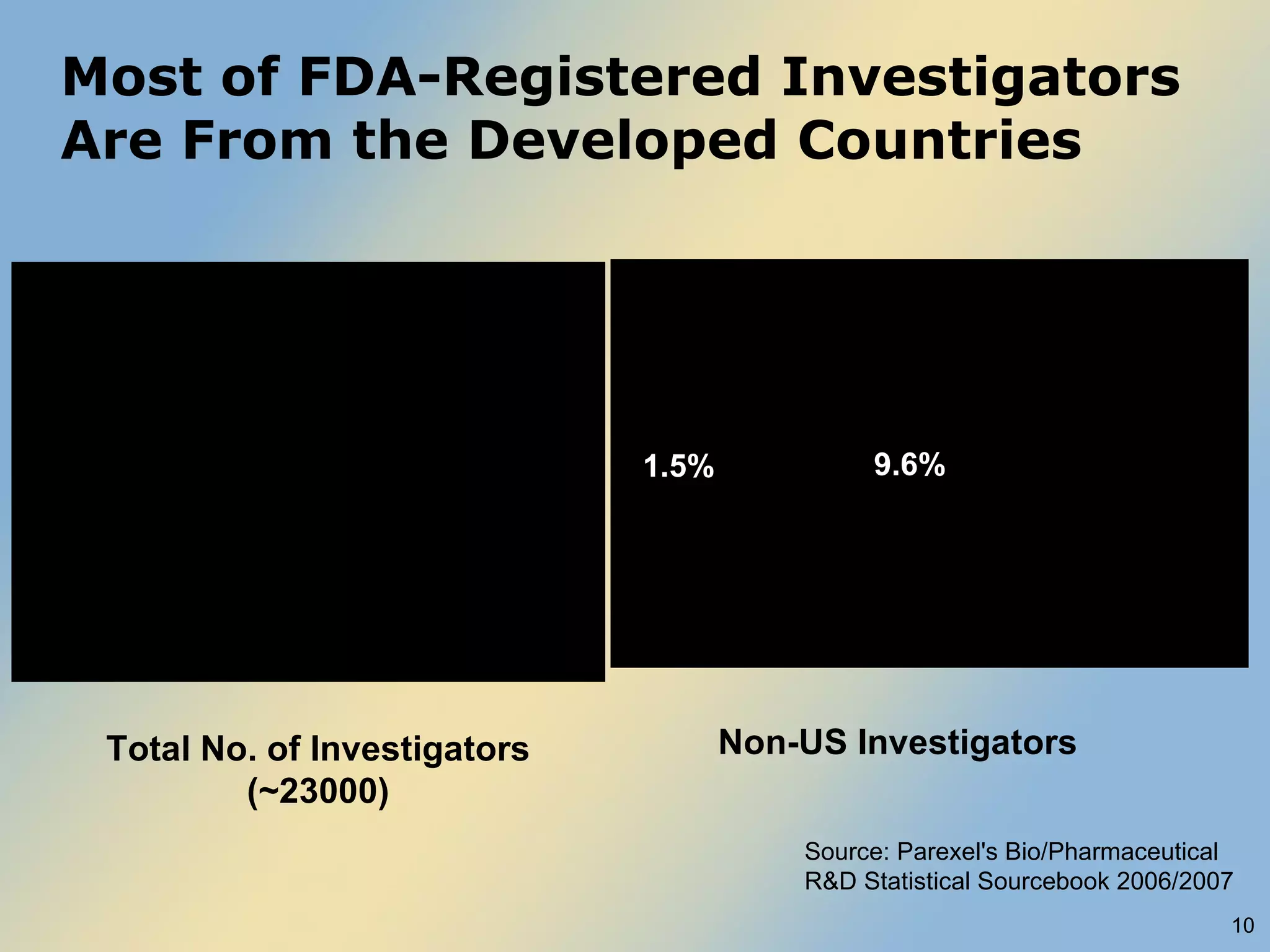
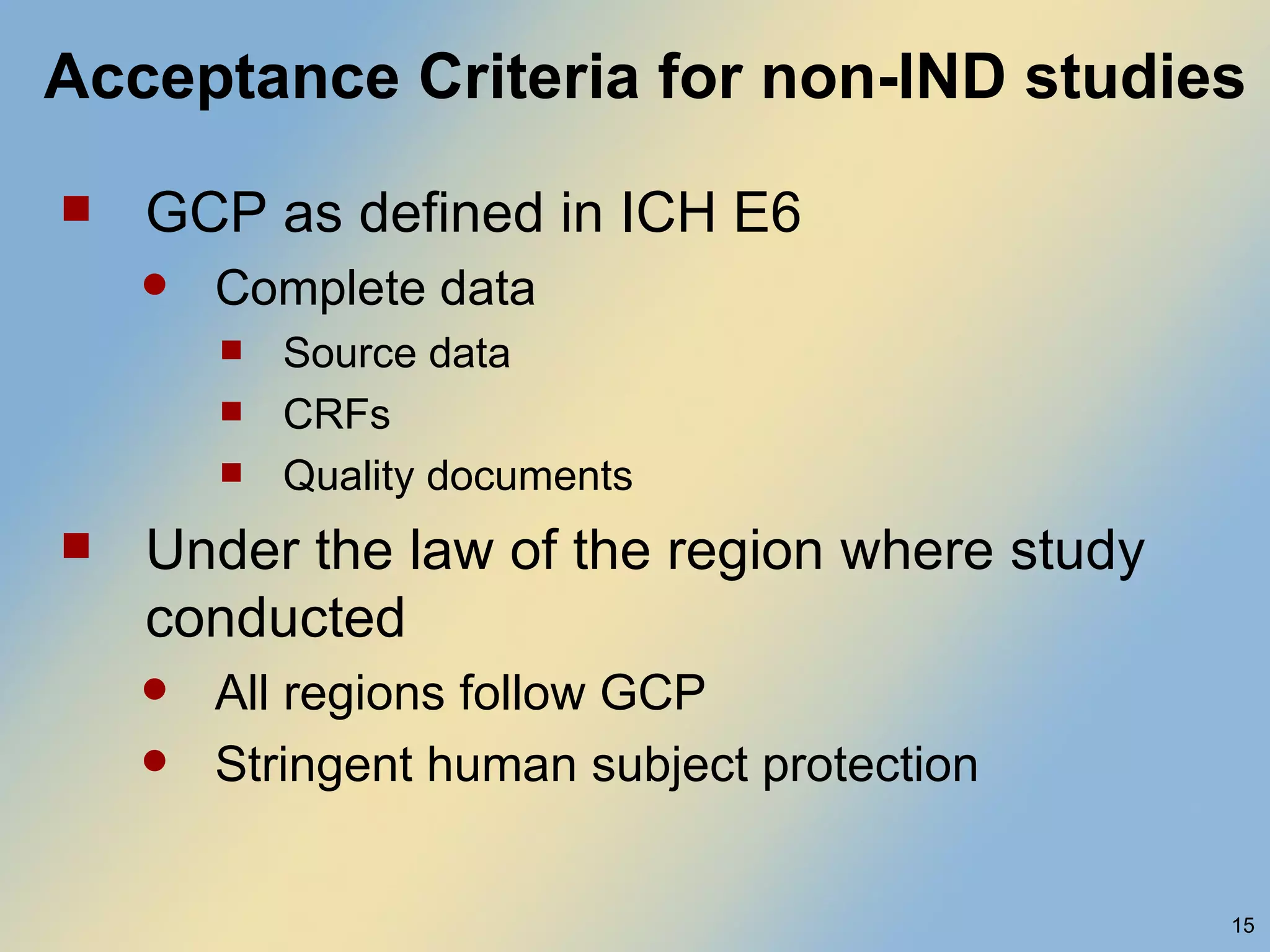
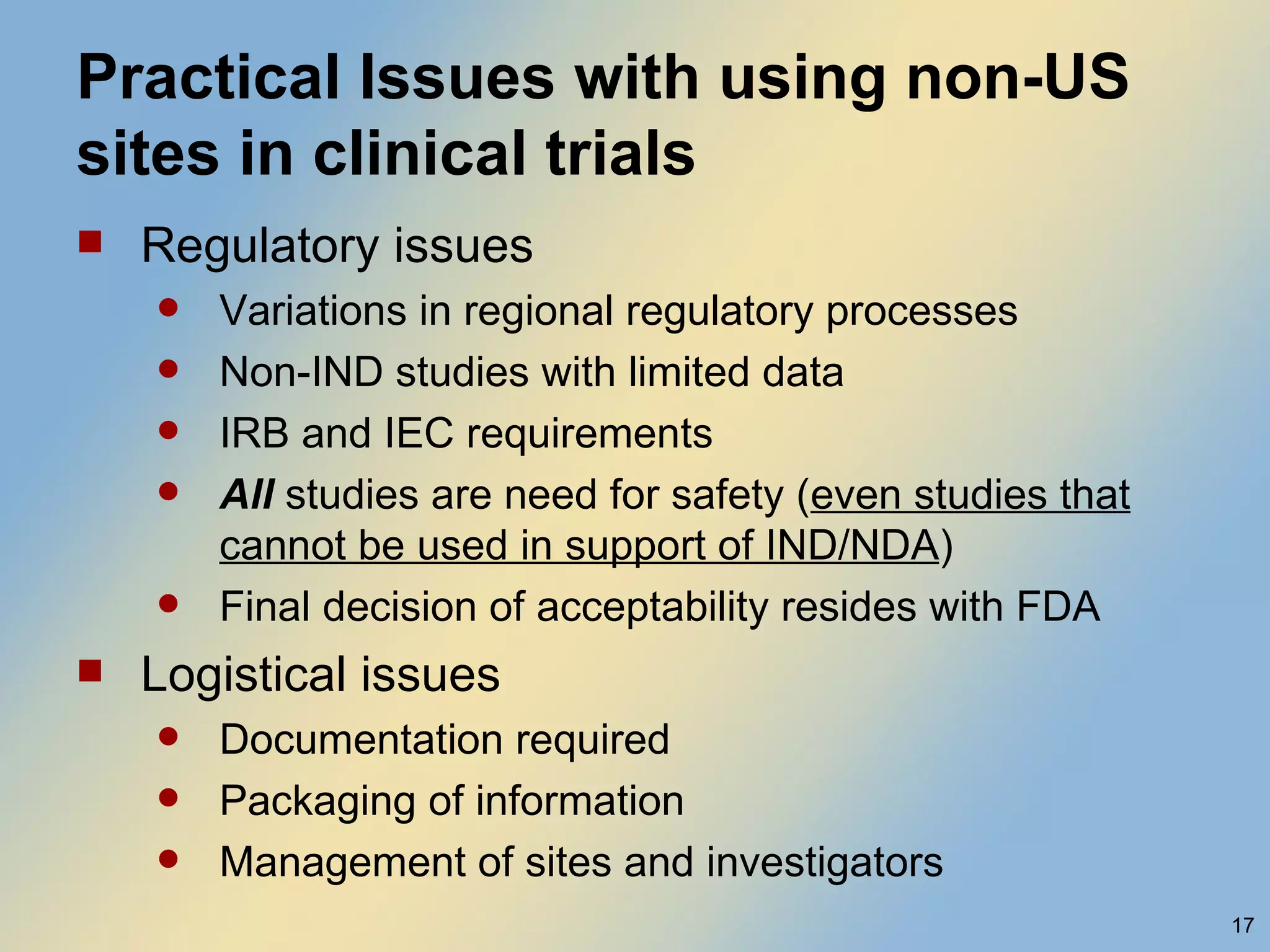
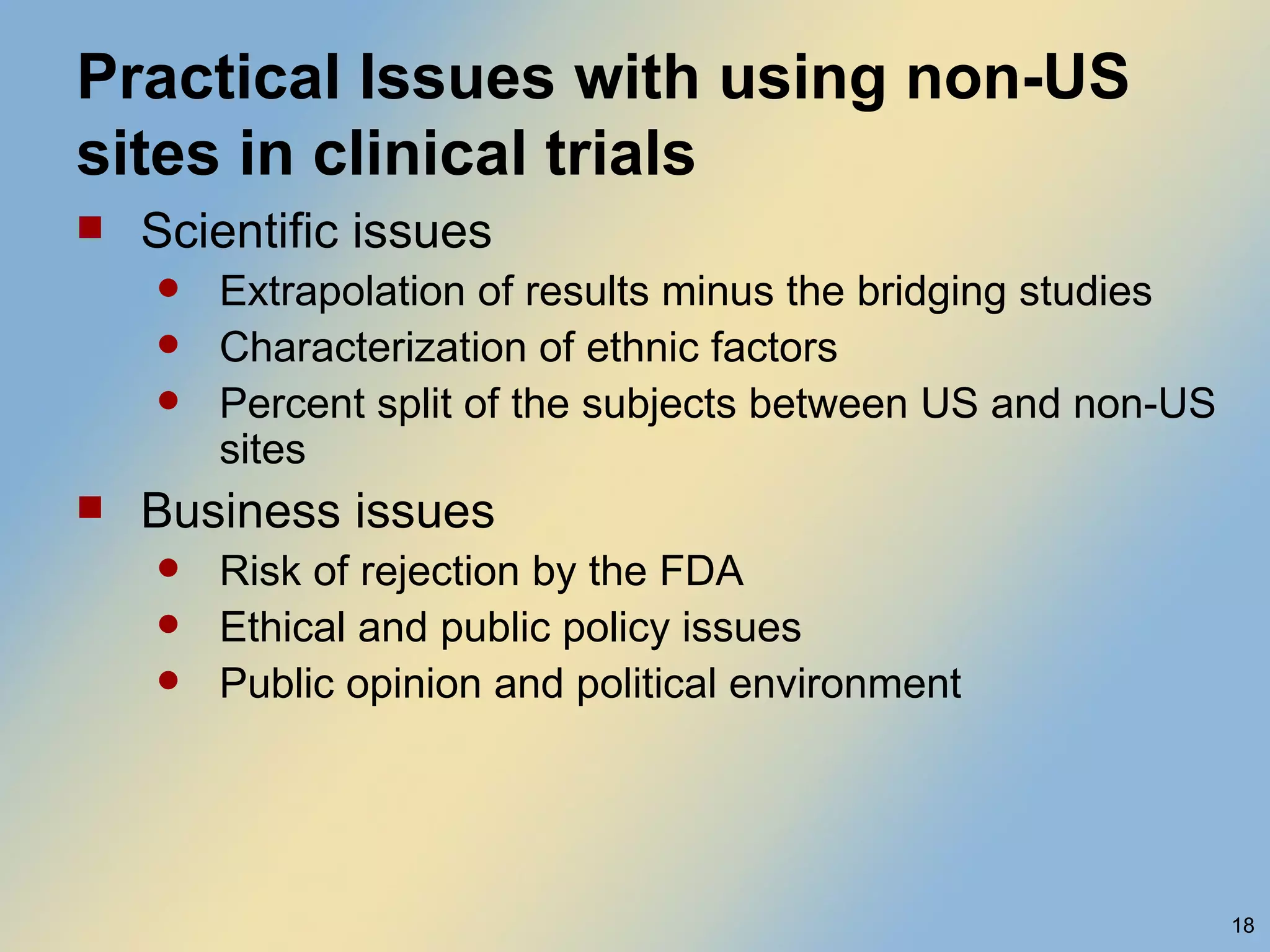
The document discusses FDA acceptance of foreign clinical trial data for regulatory submissions. It provides an overview of current regulations and considerations for both IND and non-IND studies conducted abroad. Key points include that foreign data is acceptable if studies meet good clinical practices and are applicable to the US population. While IND studies must follow all US rules, non-IND studies require proof of GCP compliance and complete documentation for FDA to consider acceptance. Practical and scientific issues in extrapolating results from foreign to US populations are also addressed.
![A Product Development Services Company From Lab to Market Approval Regulatory Strategy Strategy Implementation Pre-Clinical Assays Global Clinical Trials Global Regulatory Submissions Contact: Patrick Burke at [email_address] 301-528-7000 Amarex Clinical Research Washington DC metro area](https://image.slidesharecdn.com/FDAAcceptanceofForeignClinicaltrialDataFeb2009-123550352581-phpapp01/75/Fda-Acceptance-Of-Foreign-Clinical-Trial-Data-Feb-2009-1-2048.jpg)

![FDA Acceptance of Foreign Clinical Trial Data in INDs and NDA/BLA Mukesh Kumar, PhD, RAC Senior Director, Regulatory Affairs Amarex Clinical Research Germantown, MD 20874, USA Tel: 001-240-750-4893 Email: [email_address]](https://image.slidesharecdn.com/FDAAcceptanceofForeignClinicaltrialDataFeb2009-123550352581-phpapp01/75/Fda-Acceptance-Of-Foreign-Clinical-Trial-Data-Feb-2009-3-2048.jpg)











![Thank You! Questions and Comments Mukesh Kumar, PhD, RAC Senior Director, Regulatory Affairs Amarex Clinical Research Germantown, MD 20874, USA Tel: 001-240-750-4893 Email: [email_address]](https://image.slidesharecdn.com/FDAAcceptanceofForeignClinicaltrialDataFeb2009-123550352581-phpapp01/75/Fda-Acceptance-Of-Foreign-Clinical-Trial-Data-Feb-2009-21-2048.jpg)