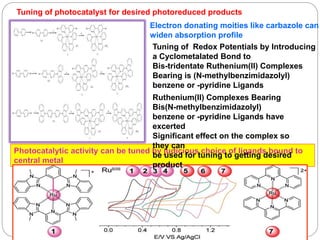
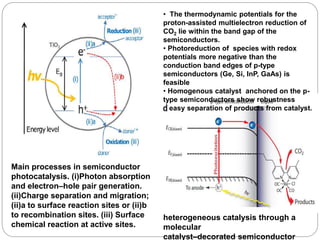
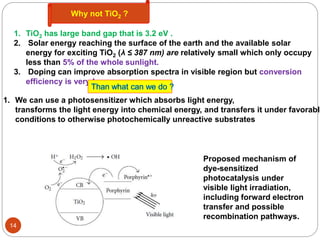
This document provides an overview of homogenous photocatalytic reduction of CO2. It discusses key topics such as what photocatalysis is, problems with CO2 reduction, classifications of photocatalysts including homogeneous and heterogeneous examples, and mechanisms of type I and type II catalysts. Molecular complexes like rhenium and ruthenium are described as promising homogeneous photocatalysts. The effects of catalyst structure, reaction conditions, and anchoring to surfaces are reviewed. Future areas of improvement include increasing turnover numbers and standardizing test conditions for fair catalyst comparisons.

![7
Terminology :
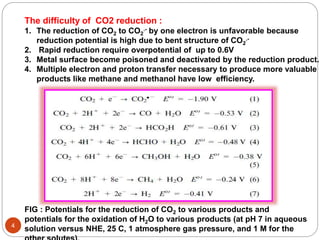
Catalytic selectivity (CS) = [CO2 reduction products] / [other
product and
H2 ]
Quantum Yeild = [CO2 reduction products] / [incident
photons]
Turnover Number (TN) = [CO2 reduction products]
/[catalyst]
Overpotential = Applied potential – Thermodynamic
potential
Turn over frequency (TOF) = Catalytic turn over per unit time
Faradaic effeciency (FE) = [Product]/[electron passed] x number of electron
needed for conversion](https://image.slidesharecdn.com/pawan-170411204224/85/Pawan-Homogeneous-catalyst-for-CO2-reduction-7-320.jpg)
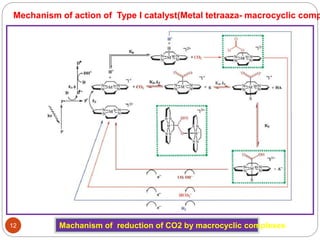
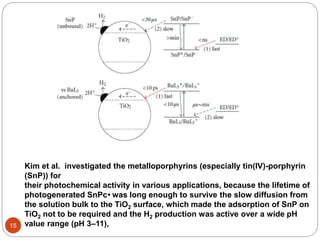
![(i)Type I photocatalysis :
P + hν = P*
P* + Et3N = P- + Et3N•+
P- + cat = P + cat-
cat- + CO2 = cat + products
Et3N•+ + Et3N = Et3NH+ + Et2NC•HCH3
Et2NC•HCH3 + P (or cat) =
Et2N+CHCH3
+ P- (or cat-
)
10
Tertiary amine known as the “sacrifice
reagent”. Theses provides electrons to
sensitizer for further reaction
TEA, TEOA, and [Co(NH3)5Cl]2+ have been
shown to readily decompose once the
electron transfer has taken place, thus
preventing any further non-productive
back reactions
1-benzyl-1,4-dihydro-
nicotinamide
Types of molecular homogenous photocatalsts :
“A molecular light absorber and
transition metal complex works in
concert, without one there will be
no reaction.”
eg : (a) [Ru(bpy)3]2+
(photosensitizer) and Re
complexes (catalyst)
(b) Metal tetraaza-macrocyclic
complexes
(c) Supramolecular complexes](https://image.slidesharecdn.com/pawan-170411204224/85/Pawan-Homogeneous-catalyst-for-CO2-reduction-10-320.jpg)
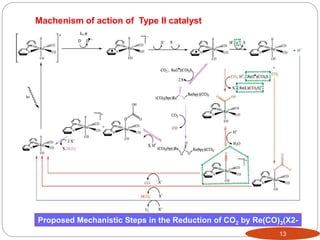
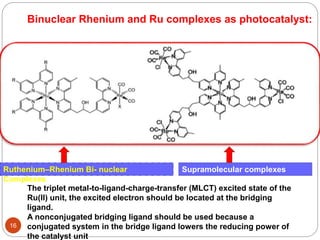
![Type II photocatalysis : “One single compound acts as both the light
absorber and the catalyst.”
eg: (a)Metal porphyrin derivates
investigated for CO2 reduction
(b) Re[(CO)3(bpy)]X based complexes
metalloporphyrin (MP), metallocorrin (MN), metallophthalocyanine
(MPc), and metallocorrole (MC, where R ) C6F5 or 2,6-C6H3Cl2).11
Pcat + hν = Pcat*
Pcat* + Et3N = Pcat- + Et3N•+
Pcat- + CO2 = Pcat + products
Et3N•+ + Et3N = Et3NH+ + Et2NC•HCH3
Et2NC•HCH3 + Pcat = Et2N+ CHCH3 +
Pcat-](https://image.slidesharecdn.com/pawan-170411204224/85/Pawan-Homogeneous-catalyst-for-CO2-reduction-11-320.jpg)
![24
Comparison of UV-Vis spectra of Ru(bpy)3
2+ in high purity water and
acetonitrile (AN). The volume ratio between the amount of acetonitrile to
dissolve Ru(bpy)3
2+ powder and the water to prepare the solution was 1:100.
The spectra were recorded after the solutions reached equilibrium.
[Ru(bpy)3
2+ ] = 1.108 x 10-5 M.
Effect of solvent on absorbance of Ru complex4.
Photocatalytic activity get increased in non-aqueous solvent in comparison to a
media because CO2 have more solubility(7-8 time ) in Organic solvent
eg: Acetonitrile, DMF, DMSO etc.](https://image.slidesharecdn.com/pawan-170411204224/85/Pawan-Homogeneous-catalyst-for-CO2-reduction-24-320.jpg)