The document is a project report on the green synthesis of zinc nanoparticles using Eclipta alba leaf extract. It includes an introduction describing nanoparticles and zinc nanoparticles. It then discusses the objectives, literature review, and methodology of the project. The methodology section describes the preparation of zinc nanoparticles using the leaf extract. It also describes characterization using UV-Vis spectroscopy and testing antimicrobial activity against E. coli and Staphylococcus aureus. The results and discussion section analyzes the UV-Vis spectra and effect of various parameters on synthesis. It finds the green zinc nanoparticles show improved antimicrobial activity against the tested bacteria.










![INTRODUCTION
1.1 Background
There are several interesting examples in nature where nanostructures are
present and have important functions. In recent years, there is much growing concern
towards the eco-friendly production of nanoparticles because of their novelty that
make them feasible for various potential applications in different areas of science and
technology (Borase et al. 2014). The outstanding progress of nanoscience and
technology is the part and parcel of advancement in measurement systems, method
and instruments. The recent trend of increased interest from bulk to nanotechnologies
raises a number of new explicit problems due to the small dimensions and structures
which needs to be addressed and explored in this area. Particularly, in nanotechnology
the hypothesis applies: “If you can’t measure it accurately, you can’t construct it and
reproduce it in number of times.” Nanotechnology may be the subsequently huge
craze in science, and before that we will be possibly find ourselves immersed in it [1].
The idea of nanotechnology though appraise to be a recent science has its
antiquity dating to as back as the 9th century. The artisans of Mesopotamia used gold
and silver nanoparticles to produce an impressive effect to pots. The initial scientific
explanation of the significance of nanoparticles was given in 1857 by Michael
Faraday in his popular paper “Experimental relations of gold (other metals included)
to light” (Faraday, 1857). In 1959, Richard Feynman a talked about explained
molecular machines built with atomic accuracy. This was considered the initial talk
on nanotechnology. This was entitled “There’s plenty of space at the bottom”.
Nanotechnology can be termed as the synthesis, characterization, investigation and
application of nanosized (1-100 nm) resources for the expansion of science [1, 2]. It
deals with the resources whose structures reveal considerably new and enhanced
physical, chemical, and biological properties, phenomena, and functionality
appropriate to their nano scaled range. Because of their size, nanoparticles have a big
surface part than macro-sized materials. The essential properties of metal
nanoparticles are mostly determined by range, shape, composition, crystalline and
morphology (Ranjan et al. 2009). Nanoparticles outing to their small size, have
diverse properties compared to the mass form of the same substance, thus contribution
many novel developments in the fields of biosensors, biomedicine, and bio](https://image.slidesharecdn.com/e1965f4b-dc32-4e19-a222-5689bda8cedd-150531162916-lva1-app6892/85/nanotechnology-11-320.jpg)
![nanotechnology. Nanotechnology is furthermore human being utilized in medicine for
diagnostic, therapeutic drug delivery and the improvement of treatments for many
diseases and disorders. Nanotechnology is an a great deal potent technology, which
holds an enormous promise for the design and development of many kinds of novel
product with its prospective medical applications on untimely disease detection,
treatment, and prevention. (Gopinath et al.2012)[2].
1.2 Nanoparticles
The word “nanoparticles” that is used to express a particle with extent in the
range of 1nm-100nm, at slightest in one of the three possible dimensions. Inside this
size range, the physical, chemical and biological properties of the nanoparticles
changes in fundamentals conduct from the properties of both being atoms/molecules
and of the equivalent bulk materials (Nour et al. 2010)[3]. Nanoparticles can be
prepared of materials of various chemical nature, the largely frequent being metals,
metal oxides, silicates, non-oxide ceramics, polymers, organics, carbon and
biomolecules. Nanoparticles are present in numerous changed morphologies such as
spheres, cylinders, platelets, tubes etc. Generally the nanoparticles are considered
through surface modifications adapted to gather the needs of specific applications
they are going to be used for. The enormous diversity of the nanoparticles arising
from their wide chemical nature, shape and morphologies, the medium in which the
particles are present, the state of dispersion of the particles and most importantly, the
numerous possible surface modifications the nanoparticles can be subjected to make
this an important active field of science now-a-days (Zamiri et al.2012)[3].
1.3 Unique properties of nanoparticles
A number of physical phenomena become more definite as the size of the
system reduced. Some phenomena may not be significant as we move from macro
(high) to micro (low) stage but may be notable at the nano scale. Another example of
uniqueness of nanoparticle is that when we increase the surface area with respect to
volume changes the catalytic, mechanical and thermal and properties of the material.
Also it increases of the superiority in the behaviour of atoms on the surface of the
particle over the atoms in the interior, thereby altering the properties. The properties
(optical, electrical properties and chemical reactivity) of small clusters are different](https://image.slidesharecdn.com/e1965f4b-dc32-4e19-a222-5689bda8cedd-150531162916-lva1-app6892/85/nanotechnology-12-320.jpg)
![from the properties of those components in bulk. Also there are size dependent
properties of nanoparticles that are quantum incarceration in semiconductors, in
metallic nanoparticles surface Plasmon resonance and paramagnetic in magnetic
nanoparticles [4]. In resonance, the collective oscillations of the conduction of
electrons with the light field are known as surface Plasmon resonance. This property
emerges from the electron confinement in the nanoparticles. The frequency also
depends on the shape and size of the nanoparticles,the dielectric properties of the
medium surrounded and also the metal (Jain et al. 2007). For instance, the noble
metals like gold and silver nanopartiles reveal unique and tuneable optical properties
because of their surface Plasmon resonance.
1.4 Zn nanoparticles and its current status
Zn appears as a white powder and is practically insoluble in water. Zn is one
of the most significant semiconducting materials with exclusive properties and
resourceful applications. Zn has a stable quartzite structure consisting of a number of
planes composed of tetrahedral coordinated O2− and Zn2+ ions. Because of its no
central symmetry, Zn is piezoelectric, which is a key property in building
electromechanical coupled sensors and transducers (Albrecht et al.2006). Zinc
nanoparticles can be present in ions only in the existence of strong oxidizing
substances. Zn is an environmental friendly material and biocompatible which is
desirable especially for biomedical applications. Zinc nanoparticles have received
considerable attention due to their antimicrobial, UV blocking, high catalytic and
photochemical activities. Zn is non-hazardous and is compatible with human skin
building it a proper additive for textiles and surfaces that get nearer in contact with
human body (Li et al. 2011). The increase in surface area of nanoscale Zn compared
to immensity has the potential to develop the effectiveness of the material function.
Zinc is a natural constituent and an intrinsic part of the environment. Exposure to
natural environment levels of zinc in the biosphere is crucial for all living organisms,
fulfilling important metabolic functions in humans, animals and plants. Although zinc
oxide is generally recognized as safe, occupational, consumer, and environmental
exposures are controllable and considered to pose little or no risk. Zinc occurs
naturally in soil, sediment, water and air, from where it is taken up by organisms to
fulfil essential functions in metabolism. As such, the distribution, transport and effects](https://image.slidesharecdn.com/e1965f4b-dc32-4e19-a222-5689bda8cedd-150531162916-lva1-app6892/85/nanotechnology-13-320.jpg)
![(bioavailability) of zinc in the environment largely depend on the site‐specific
physicochemical characteristics of the environment and an organism’s condition. Zn
has received much attention from researchers and regulatory agencies due to its use in
cosmetics and personal care products [5]. However, consumer and environmental
safety interests have been demonstrated through various lines of evidence, including
endorsed use of nano. Zn containing sunscreens by the U.S. FDA (Food and drug
Administration) and Australian TGA (Therapeutic goods Administration) Following
several assessments on potential occupational, consumer, and environmental exposure
and effects, it has been demonstrated that the levels of nano Zn present in cosmetics,
effluents, or environmental media do not pose health risks to humans.
1.4.1 Viable applications of Zn nanoparticles
1. It is used in paints, cosmetics, sunscreens, plastic and rubber manufacturing,
electronics and pharmaceuticals products etc.
2. It is also potentially used to treat leukaemia and carcinoma cancer cell
3. It is also a physically powerful antibacterial agent.
4. It is also used as drug transporter.
5. Zn nanoparticles is also used in industrialized sectors together with
environmental, synthetic textiles, food, packaging, medical care, healthcare, as
well as construction an decoration.
6. Zinc Nanoparticles exhibited feasible applications such as therapeutics, drug
delivery agents, biosensors, imaging contrast agents, transfixion vectors, and
fluorescent labels. Plant mediated zinc nanoparticles showed best anticancer
activity.
7. It has been used as a source of zinc which is an essential trace element in food
industry.
8. It also widely applied to various cosmetic products like sunscreen products
while it acts as an invisible obstacle which scatters UV radiation away from
the skin relatively than permitting its destructive energy to be absorbed. But,
absorption rate of zinc is low via oral ingestion and bulk-sized ZnO has poor
dispersion property, resulting in low UV blocking capacity, low transparency
on skin, and hard agglomeration compared to small-sized ZnO. Thus, nano-](https://image.slidesharecdn.com/e1965f4b-dc32-4e19-a222-5689bda8cedd-150531162916-lva1-app6892/85/nanotechnology-14-320.jpg)
![sized ZnO has recently attracted much attention in order to enhance the uptake
of zinc and increase UV filtering efficiency with cosmetic clarity.
9. The similar kind of active ingredient used in the calamine lotion and diaper
cream.
10. It is now generally accepted that nanoparticles efficiently penetrate the cell
membrane compared to micro-sized particles. However, few researches were
performed to demonstrate the effects of physicochemical parameters of ZnO
nanoparticles on cellular uptake, which may provide critical information on
their toxicity potential [6].
11. Most activities to conflict and have centered on the nano coatings or nano
composites using nanoparticulates of silver and zinc oxide. Their
antimicrobial effects of natural biological compounds have also been
demonstrated and confirmed by various researches .Zinc oxide show evidence
of antibacterial activity which increases with decreasing the particle size. ZnO
is nontoxic and it is a strong antibacterial agent.
12. ZnO nanoparticles have strong resistance to microorganisms and this property
is due to the production of reactive oxygen species (ROS) on the surface of
nanoparticles.
13. Zinc nanoparticles are used as preservative for various materials and products
such as plastics, ceramics, glass, pigments, foods, etc.
1.5 Synthesis of nanoparticles
Synthesis of dimensionally controlled particles enlarges quantities and studies
their properties to investigate novel applications is a preferred activity for researchers
in nanomaterials. There are several physical, chemical procedures for synthesis of Zn
nanoparticles in large quantities in a short period of time. Among them simple-
solution based methods, chemical precipitation, sol-gel or hydrothermal,
electrochemical and photochemical reduction method are preferable methods (Seow
et al.2011). Zn nanoparticles can be synthesized using green method that exploits
various species of plant leaf extract, microorganisms like bacteria and fungi. Further
the green synthesis of nanoparticles also governs by various enzymes. There are a lot
of different methods of Zn nanostructures preparation like metal organic vapour phase
epitaxial, evaporation of high temperature, gas spraying, pulsed laser deposition,](https://image.slidesharecdn.com/e1965f4b-dc32-4e19-a222-5689bda8cedd-150531162916-lva1-app6892/85/nanotechnology-15-320.jpg)
![sputtering, sol-gel, wet chemical and electrochemical methods. Different applications
of nanoparticles based on different protocols which have been designed for synthesis
of nanoparticles. Figure 1 summarized the different methods used for synthesis of
nanoparticles (physical, chemical and biological mathods).
Fig.1.Outline of the various approaches such as physical, chemical and biological
for the synthesis of nanoparticles
1.5.1. Physicalmethod
Physical methods developed for synthesis of Zn nanoparticles often require
special equipments or operational control. The physical method often called top-down
approach which includes methods like diffusion, irradiation, thermal decomposition
and arc discharge etc. The thermal decomposition method is one of the significant
top-down approaches. It is used for the creation of monodisperse nanoparticles. These
approaches use larger initial structures or macroscopic units which can be externally
controlled in the processing of nanostructures. Etching through the mask; ball milling
and application of severe plastic deformation are common examples of physical top-
down method of synthesis of nanoparticles[7]. The top-down method of synthesis is a
model which generates on a larger scale for further reduction of nano scale. Mostly
the top-down methods are not cheap and quick to manufacture. This method is slow](https://image.slidesharecdn.com/e1965f4b-dc32-4e19-a222-5689bda8cedd-150531162916-lva1-app6892/85/nanotechnology-16-320.jpg)
![and not suitable for large scale production. In this method of synthesis, fatty acids are
allowed to dissolve in the hot NaOH solution and then mixed with the metal salt
solution. After that, the solution used to forms the metal precipitates. Crystals and
short wires of copper are allowed to be encircled in the glass ampoules by the
diffusion method. Then, it is sealed at low pressure followed by annealing at 5000˚C
for 24 hours. Subsequently the crystals are eradicated from the ampoules. At room
temperature, the ampoules free crystals are chilled on a metallic plate. In UV
irradiation method, polycarbonate films are placed on glass microscope slide .Then
this slide is exposed to UV radiation. Figure 2 depicts the synthesis of nanoparticles
by top down method.
1.5.2. Chemicalmethod
Moderate reaction position and suitable artificial manipulation have rendered
chemical methods a striking option for accessing Zn nanoparticles. Conventional
chemical routes that are recurrently used in modern years include sol-gel approach,
chemical precipitation and colloidal synthesis. Surfactants or polymer based
preparative method for Zn nonmaterials are also quite accepted to avoid
agglomeration and accomplish better manages of Zn nanoparticles. Removal of
surfactants or polymers, however, poses serious limitations to such methods. Hence,
delicate and precise control of experimental factors for the production of
nanoparticles with desired morphologies is a cherished goal for future device
applications [7]. Homogeneous chemical precipitation method is often considered
economically viable for preparation of mono disperse metal oxide particles of
different shapes and sizes. The method also provides better control of chemical and
morphological characteristics. Chemical methods, including precipitation from
inorganic or organic solutions and sol-gel techniques can conveniently provide
control of nucleation, growth and ageing of particles in the solution. The methods rely
on advanced solution and coordination chemistry theories; enabling the synthesis of
their quirked precursor particles utilizing a variety of parameters to enable control of
the solid formation process. A major contribution to the growth of particles is through
Ostwald ripening, where small particles with lower solubility product dissolve and re-
precipitate on the surface of larger particles in solution. Agglomeration takes place in
solution as the particles clog together to minimize surface energy. Mostly the
chemical methods of synthesis of nanoparticles are the bottom up approaches. These](https://image.slidesharecdn.com/e1965f4b-dc32-4e19-a222-5689bda8cedd-150531162916-lva1-app6892/85/nanotechnology-17-320.jpg)
![approaches include the miniaturization of material components (up to atomic level)
with further self-assembly processes leading to the formation of nanostructures.
During self-assembly the physical forces operating at nanoscale are used to combine
basic units into larger stable structures. Typical examples are quantum dot formation
during epitaxial growth and formation of nanoparticles from colloidal dispersion [8].
In these methods different metal particles are reduced to form nanoparticles. Various
types of metal particles used like Sodium monohydrate and Sodium citrate etc. Other
chemical reagents used are N, N-dimethylformamide (DMF), Poly (N-vinyl
pyrrolidine (PVP), ethyl alcohol, tetra-n-tetra-fluroborate (TFATEB), CTAB, etc.
This method initiates with a pattern which is generate larger scale, then reduced to
nanoscale[9]. This method of synthesis of nanoparticles is cheap and quick to
manufacture. This method is slow and not suitable for the large scale production.
(Figure 2 displays the chemical method for nanoparticles synthesis)
Fig.2. Synthesis of nanoparticles by physical (top down) and chemical method
(bottom up)
1.5.3. Biologicalmethod
Biosynthesis of nanoparticles is a sort of bottom up (chemical method)
approach where the main reaction taking place is reduction/oxidation. The
requirement for biosynthesis of nanoparticles increases as the physical and chemical
methods were expensive. Often, chemical synthesis method leads to formation of
some of the toxic chemical engrossed on the surface that may have unfavourable
effect in the medical applications. This is not a problem when it comes to](https://image.slidesharecdn.com/e1965f4b-dc32-4e19-a222-5689bda8cedd-150531162916-lva1-app6892/85/nanotechnology-18-320.jpg)
![biosynthesized nanoparticles via green synthesis way. So, in the investigation of
cheaper pathways for nanoparticles synthesis, scientist used microbial enzymes and
plant extracts (phytochemicals) [14]. With their antioxidant or dropping properties
they are regularly dependable for the decrease of metal compounds into their
particular nanoparticles. Green synthesis provides improvement over chemical and
physical method as it is price effective, environment friendly; easily scaled up for
large scale synthesis and in this method there is no need to use high pressure, energy,
temperature and toxic chemicals. Green synthesis technique is proved as favourable
over other techniques (physical and chemical methods) which are implemented for the
production of nanoparticles. Green synthesis exploitation of biological materials like
plant leaf extract, bacteria, fungi and enzymes methods are eco-friendly, advance and
compatible for pharmaceutical and other biomedical applications, as the toxic
chemicals are not used in these methods. As well, this method does not require of
high pressure and temperature.
1.6. Advantages of Zn nanoparticles synthesis by plants over
microbes
The major advantage of using plant extracts for zinc nanoparticle synthesis is
that they are simply accessible, safe, and non-hazardous in generally cases, have a
broad multiplicity of metabolites that can assist in the reduction of zinc ions, and are
faster than microbes in the synthesis because it’s not easy to maintenance of
microbial cell culture and contamination [12]. The main method considered for the
procedure is plant-assisted reduction due towards phytochemicals. The major
phytochemicals concerned are, ketones, aldehydes, terpenoids, carboxylic acid
flavones and amides. Organic acids, Flavones and quinones are water-soluble (soluble
in water) phytochemicals that are dependable for the instant reduction of the ions.
Studies have exposed that xerophytes have emodin, an anthraquinone that undergoes
tautomerization, most important to the formation of the zinc nanoparticles. Inside the
case of mesophytes, it was founds that they hold three types of benzoquinones:
cyperoquinone, dietchequinone, and remirin. It was optional to directly implicate the
phytochemicals in the reduction of the ions and productionof zinc nanoparticles[16].](https://image.slidesharecdn.com/e1965f4b-dc32-4e19-a222-5689bda8cedd-150531162916-lva1-app6892/85/nanotechnology-19-320.jpg)

![REVIEW OF LITERATURE
The study about Nanoscience and nanotechnology provides the well developed
application of exceptionally miniature things and be capable of the encroachment of
all the fields of scientific research and development like Physics, Chemistry,
Materials and Metallurgy engineering, Biology and also in Biotechnology.
Bionanotechnology is the conjunction between biotechnology and nanotechnology for
developing various biosynthetic and environmental eco-friendly technologies for the
synthesis of various nanomaterials. Multiple of research on synthesis of nanoparticles
has put forth great interest towards the emerging field of science due to their
distinguishing physical and chemical properties than the macroscopic particles. The
vast array of development of novel synthesis protocols and various characterization
techniques are the evidences for the vast advancement for the nanotechnology.
A critical review of literature depicted that currently, the nanobiotechnology is an
interesting emerging and innovative field of research with possibility to explore
various potential application. Taking this facts into consideration, now a days there is
intensifying research towards the same area. Further the scientific/researcher
commitments actively engage in the same field to drive and bring this area of research
from infancy to maturity level.
Jalal et al (2010) studied on “Zn nanofluids: Green synthesis,
characterization, and antibacterial activity”. In this paper using a green solvent and
ionic liquid, 1-butyl-3-methylimidazolium bis (trifluoromethylsulfonyl) imide,
[bmim] [NTf2], Zinc oxide nanoparticles have been formed by microwave
degeneration of zinc acetate precursor. Using transmission electron microscopy and
X-ray diffraction, the structure and morphology of Zn nanoparticles have been
characterized. The Zn nanofluids have been formulated by diffusing Zn nanoparticles
in glycerol as a base fluid using a dispersant as ammonium citrate. The antimicrobial
activity of diffusion of Zn nanofluids against (E. coli) has been assessed by roughly
calculating the reducing percentage of the bacteria tested with Zn. Survival ratio of
bacteria decreases with the increase in the concentration of Zn nanofluids and also
with time. The final results show that an enhancement in the concentrations of Zn
nanofluids produces strong antimicrobial activity regarding E. coli.](https://image.slidesharecdn.com/e1965f4b-dc32-4e19-a222-5689bda8cedd-150531162916-lva1-app6892/85/nanotechnology-21-320.jpg)













![3.12 Antimicrobial activity
The antimicrobial activities of zinc nanoparticles. Where tested using
likes Staphylococcus aureus (KT68) Lot number: 11118 and Escherichia coli strain B
(KT45/45A) Lot number: #25029 by well diffusion method. Before experiment, bacterial
strain was cultured on to nutrient broth prepare by Add 1.3 g of nutrient broth powder
to 100 ml of distilled water and mix well on the other hand. Then a nutrient- broth
was prepared; the pH was adjusted to 7.0-7.5 and autoclaved. [17] It was incubated
overnight at 370C. The nutrient broth was poured into Petri dish and then master
culture was sub cultured onto nutrient agar Petri dish and it was incubated overnight
at 370 C and each strain was inoculated separately into culture broth. The culture Petri
dish was incubated overnight at 37°C in shaker-incubator. Afterwards nutrient agar
plate was arranged and punctured for wells and detect for 200uL of ZnNPs. The
labelled well was loaded with samples of Zn naoparticles. This step was carried out
for the Staphylococcus aureus, Escherichia coli bacterial strains for 6 replicates. The
plates were incubated for 24h in incubator at 37°C before the results were observed.](https://image.slidesharecdn.com/e1965f4b-dc32-4e19-a222-5689bda8cedd-150531162916-lva1-app6892/85/nanotechnology-35-320.jpg)
![RESULTS AND DISCUSSION
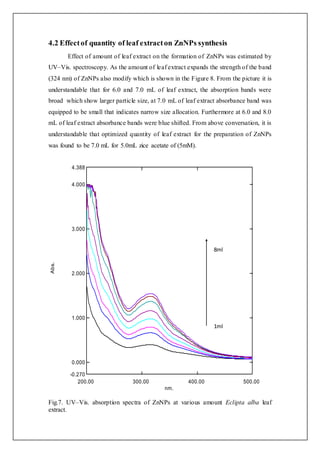
4.1 Effectof concentrationof Zinc acetate on synthesis of ZnNPs
The impact of concentrations of zinc acetate on the formation of ZnNPs was
monitored by using UV–Vis. spectroscopy. It is seen from the figure.6, at 1 mM zinc
acetate larger SPR band eventuate at 324 nm that is a distinguishing band of ZnNPs
with large size. At 3mM, zinc acetate the SPR small band at 324 nm designates minor
size of ZnNPs. For 5mM zinc acetate lager SPR band acquired at 346 nm which is
blue shift and that indicates large size distribution of ZnNPs. From the above
conversation it is understandable that in the present investigation, a most favourable
concentration of precursor for the production of ZnNPs by using Eclipta alba leaf
extract (5 mL) is found to be 5mM. Zinc acetate related explanation was also reported
by Kumar et al.[18]
Fig.6. UV–Vis. absorption spectra of ZnNPs at different concentrations of zinc
acetate
nm.
200.00 300.00 400.00 500.00
Abs.
3.595
3.000
2.000
1.000
0.000
-0.307
Zincacetate solution
5mM
1mM](https://image.slidesharecdn.com/e1965f4b-dc32-4e19-a222-5689bda8cedd-150531162916-lva1-app6892/85/nanotechnology-36-320.jpg)
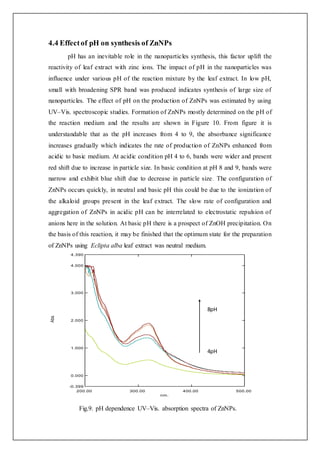
![4.3 Effectof temperature on synthesis of ZnNPs
Temperature plays one of the most significant physical parameter on the production
of Zinc nanoparticles. Figure 8 indicate the effect of temperature in the nanoparticles
production. The synthesis of nanoparticles was expanding while increasing the
reaction temperature by using the Eclipta alba leaf extract. The absorbance band was
intensify and placed at 320nm and 345 nm at the temperature of 20 °C and 60 °C,
correspondingly. The elevated rate of reduction was arising at higher temperature due
to the expenditure of zinc ions in the configuration of nuclei whereas the secondary
reduction was stopped up on the surface preformed nuclei.[19] The increase peak was
procured at low temperature indicate the development of large sized nanoparticles and
the narrow peak was procured at high temperature, which performed the synthesis of
nanoparticles are small in dimension and the higher speed of reduction of zinc ions
was occurred in the 60°C. At the end, it was summarized that high temperature was
most favourable for nanoparticles synthesis.
Fig.8. Effectof temperature inthe nanoparticlessynthesisusingleaf extractof Eclipta alba.
nm.
200.00 300.00 400.00 500.00
Abs.
4.267
4.000
3.000
2.000
1.000
0.000
-0.226
800
C
200
C1000
C](https://image.slidesharecdn.com/e1965f4b-dc32-4e19-a222-5689bda8cedd-150531162916-lva1-app6892/85/nanotechnology-38-320.jpg)
![4.5 Time dependant synthesis of ZnNPs
The previous works also recommended that contact or incubation period also
influence the synthesis of nanoparticles. This is the time interval required for
accomplishment of all steps of the reaction. Dwivedi and Gopal described the
enhancement in the sharpness of UV absorption spectrum peaks with an amplify in
contact time while functioning with plant leaf extract [20]. They revealed that
nanoparticles visible within 15 min of the reaction and increased up to 2 h, but
subsequent to that only slight variation occurred. In a distinct study, Dubey et al.
discover that in Tansy fruit-mediated synthesis the development of zinc and silver
nano- particles started contained by 10 min of the reaction. In insertion, they found
that the enhancement in contact time is dependable for the sharpening of the peaks in
equally zinc and silver nanoparticles. They also declare an important lower contact
time necessity in comparison to previous reports from Fayaz et al. and Shaligram et
al. In addition, newly Vermin et al. known that due to the instability of nanoparticles
produced an optimum period is necessary for full nucleation and consequent stability
of nanoparticles. This investigate group experiential that the optimum time essential
for the finishing point of the reaction for silver nanoparticle production was 60 min
during their testing.[21] Correspondingly, Ghoreishi et al. also showed the condition
of an optimum reaction period for the permanence of synthesized silver and gold
nanoparticles by Rosa damascene.
The impact of reaction period is shown in Fig.10.From figure and optical observations
it is understandable that the colour of solution changes after insertion of extract into
the zinc acetate solution and the aqueous solution of zinc acetate turned colourless to
brown within 5 min. SPR strength of ZnNPs enhances with instant time and saturates
within 75 min, this effect recommend the development of anisotropic molecules that
are stabilized afterwards in the medium. Numerous researchers have described on the
synthesis of ZnNPs by using different plant extract and time interval from some days
to hours, normally time duration was 7 days, 4 and 2 hours correspondingly. In the
present investigation the rate of synthesis of ZnNPs by using Eclpita alba a leaf
extract was establish to be 5 min which is very high-speed as compared to above
described plant extract, it may be due to the existence of alkaloid, terpenoid and poly
phenolic compounds such as ruin and apigenin-7-glucoside in Eclipta alba leaf
extract which acts as well-built reducing agent.](https://image.slidesharecdn.com/e1965f4b-dc32-4e19-a222-5689bda8cedd-150531162916-lva1-app6892/85/nanotechnology-40-320.jpg)
![Fig.10.Time dependant UV–Vis. absorption spectra of ZnNPs
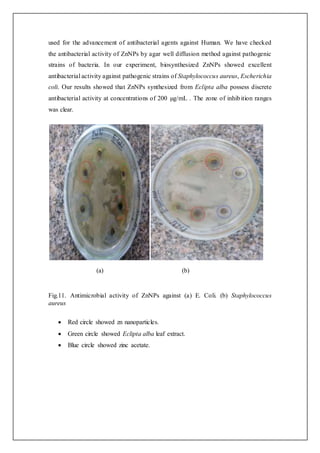
4.6 Antimicrobial activity
The ZnNPs shows exceptional antimicrobial action against E. coli. Clear area
of inhibition of bacterial expansion on nutrient agar dishes approximately the holes
impregnated with analysis ZnNPs are shown in Fig.12. The radial distance of
inhibition zone is 10 mm at the similar point the controlled cavities containing Eclipta
alba leaf extract does not exposed any inhibition zone.
The inhibitory action of Zinc compounds and Zinc ions had been traditionally
documented and applied as a helpful beneficial agent for preventing injury infections.
The inhibitory activity of zinc on bacterial cells is linked to the strong connections of
zinc with phenol groups present in key respiratory enzymes in microorganisms.
Whereas Nano crystalline Zinc shows the most efficient inhibitory activity with a fast
inhibition rate [22]. In the present study Eclipta alba was taken for synthesis of
ZnNPs because of its therapeutic values. Different studies have been done by various
researchers which verify that Eclipta alba was found to be good quality antimicrobial
nm.
200.00 300.00 400.00 500.00
Abs.
4.389
4.000
3.000
2.000
1.000
0.000
-0.415
0 min
75 min](https://image.slidesharecdn.com/e1965f4b-dc32-4e19-a222-5689bda8cedd-150531162916-lva1-app6892/85/nanotechnology-41-320.jpg)
![agent adjacent to pathogenic and non-pathogenic organisms. The antimicrobial
property of the bio-synthesized Zinc nanoparticles from Eclipta alba were also
effectively investigated. Also there is a variety of information which has been
provided that the evidences that Zinc nanoparticles were used as influential device in
opposition to multidrug-resistant bacteria. Kora and Arunachalam conceded that zinc
nanoparticles synthesized by UV photo-reduction technique are performance capable
antibacterial activity on P. aeruginosa at a great deal poor concentrations. As these
above information suggests that together Zinc nanoparticles and Eclipta alba plant
extract have exposed good quantity of antibacterial activity. Durairaj et al.
premeditated the antibacterial activity of purchased ZnNPs in (size 20-30nm)
adjacent to 10 separates of P. aeruginosa incorporating of 5 MDR strains with an
inhibition region of 11 mm experiential through µg quantity of the nanoparticles. The
nanoparticles demonstrated MIC of 50µg/ml when added at the lag stage and the sub
inhibitory concentration was measured as 100 µg/ ml.[23] In our testing, when we
compared the antibacterial action of ZnNPs and plant extract, it was found that zinc
ZnNPs have exposed additional antibacterial activity than plant extracts. Our results
evidently shows that the conventional plant extract performance some antibacterial
activity but not a large amount activity as ZnNPs does beside bacterial strains yet
taken in amount 10 times more than ZnNPs . It clearly indicates that these green
ZnNPs have shown significant quantity of action than that of plant extract.
Antibacterial movement of ZnNPs considerably increases by more than 12-15% at
very minor concentration i.e. approximately 2 out of 4 strains have showing region of
inhibition comes at concentration of 4 mg/ml in example of plant extract while in case
of ZnNPs 200 µg/ml is the maximum concentration we have used[24]. Consequently
we further takings only with the results of antibacterial motion of ZnNPs. Also if we
use synthetic or purchased ZnNPs then there may be a difficulty due to chemical
mediator used. Therefore we synthesized ZnNPs by plant extract of Eclipta alba
which potentially eliminate the difficulty of chemical mediator that could occur if we
used any artificial or chemically synthesized ZnNPs, thus production nanoparticles
biocompatible with the eco-friendly move towards. The antibacterial effectiveness of
synthesized ZnNPs enhances because the use of Zinc and Eclipta alba as zinc
inhibited in nano structure which increases its surface area, thus make ZnNPs more
reactive and Eclipta alba enhances the medicinal value of ZnNPs due to its good
antibacterial efficacy. As a result, in our study, ZnNPs prepared by Eclipta alba were](https://image.slidesharecdn.com/e1965f4b-dc32-4e19-a222-5689bda8cedd-150531162916-lva1-app6892/85/nanotechnology-42-320.jpg)






![19. Shaymurat T, Jianxiu G, Changshan X, Zhikun Y, Qing Z, Yuxue L, Yichun L
(2011) Phytotoxic and genotoxic effects of ZnO nanoparticles on garlic
(Allium sativumL.): a morphological study. Nanotoxicology 1–8.
20. Shoeb M, Braj RS, Javed AK, Wasi K, Brahma NS, et al. (2013) ROS
dependent anticandidal activity of zinc oxide nanoparticles synthesized by
using egg albumen as a biotemplate. Adv Nat Sci Nanosci Nanotechnol 4:
035015.
21. Ansari SA, Husain Q, Qayyum S, Azam A (2011) Designing and surface
modification of zinc oxide nanoparticles for biomedical applications. Food
Chem Toxicol 49: 2107–2115.
22. Nair S, Sasidharan A, Rani VVD, Menon D, Nair S, Manzoor K, et al. Role of
size scale of ZnO nanoparticles and microparticles on toxicity toward bacteria
and osteoblast cancer cells. J Mater Sci Mater Med 2009; 20:S235-41.
23. Franklin NM, Rogers NJ, Apte SC, et al. Comparative toxicity of
nanoparticulate ZnO, bulk ZnO, and ZnCl to a freshwater microalga
(Pseudokirchneriella subcapitata): the importance of particle solubility.
Environ Sci Technol. 2007;41:8484–90. [Pub Med]
24. Hanley C, Thurber A, Hanna C, et al. The influences of cell type and ZnO
nanoparticle size and immune cell cytotoxicity and cytokine induction.
Nanoscale Res Lett. 2009;4:1409–20. [PubMed]](https://image.slidesharecdn.com/e1965f4b-dc32-4e19-a222-5689bda8cedd-150531162916-lva1-app6892/85/nanotechnology-50-320.jpg)