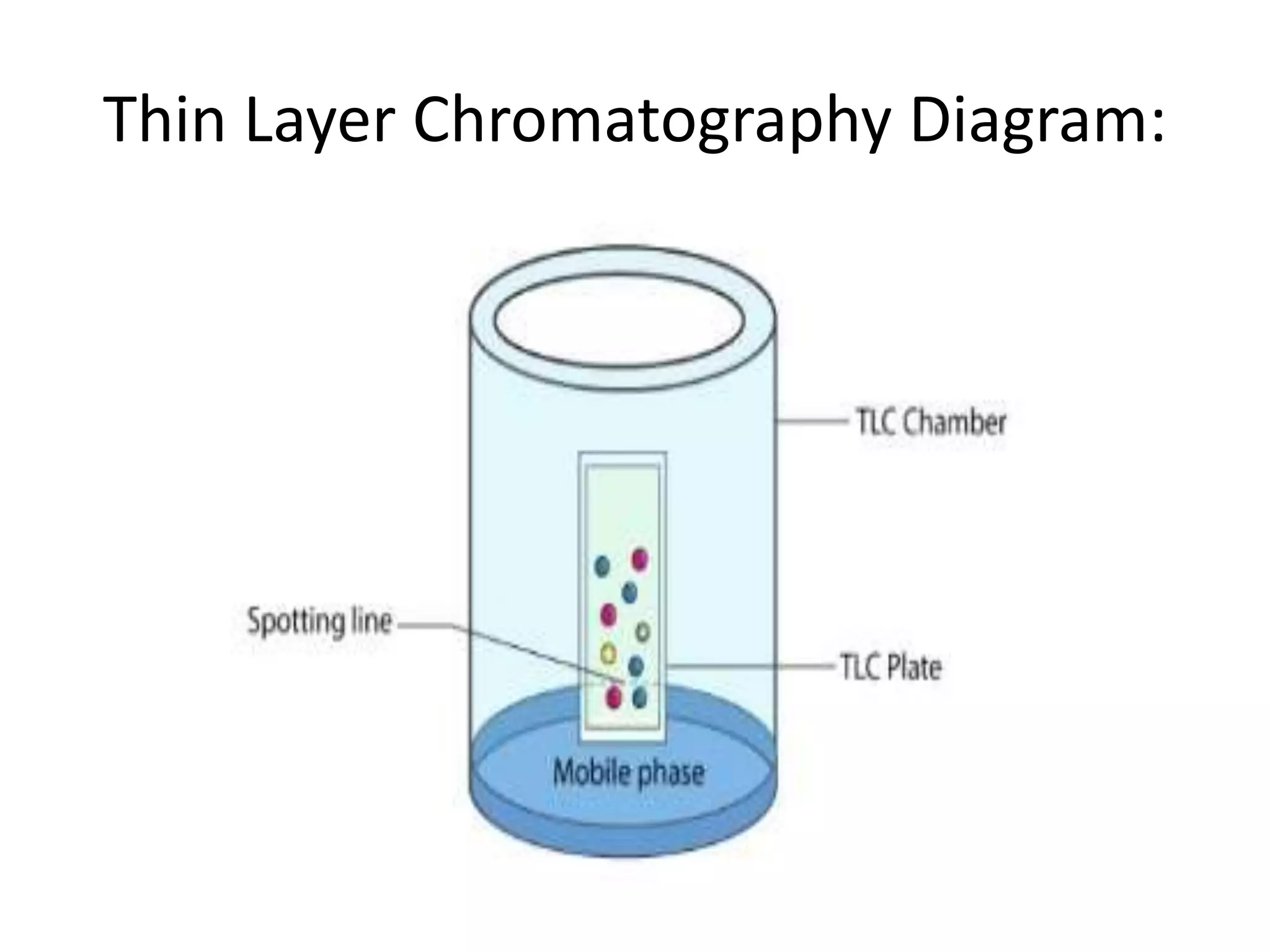
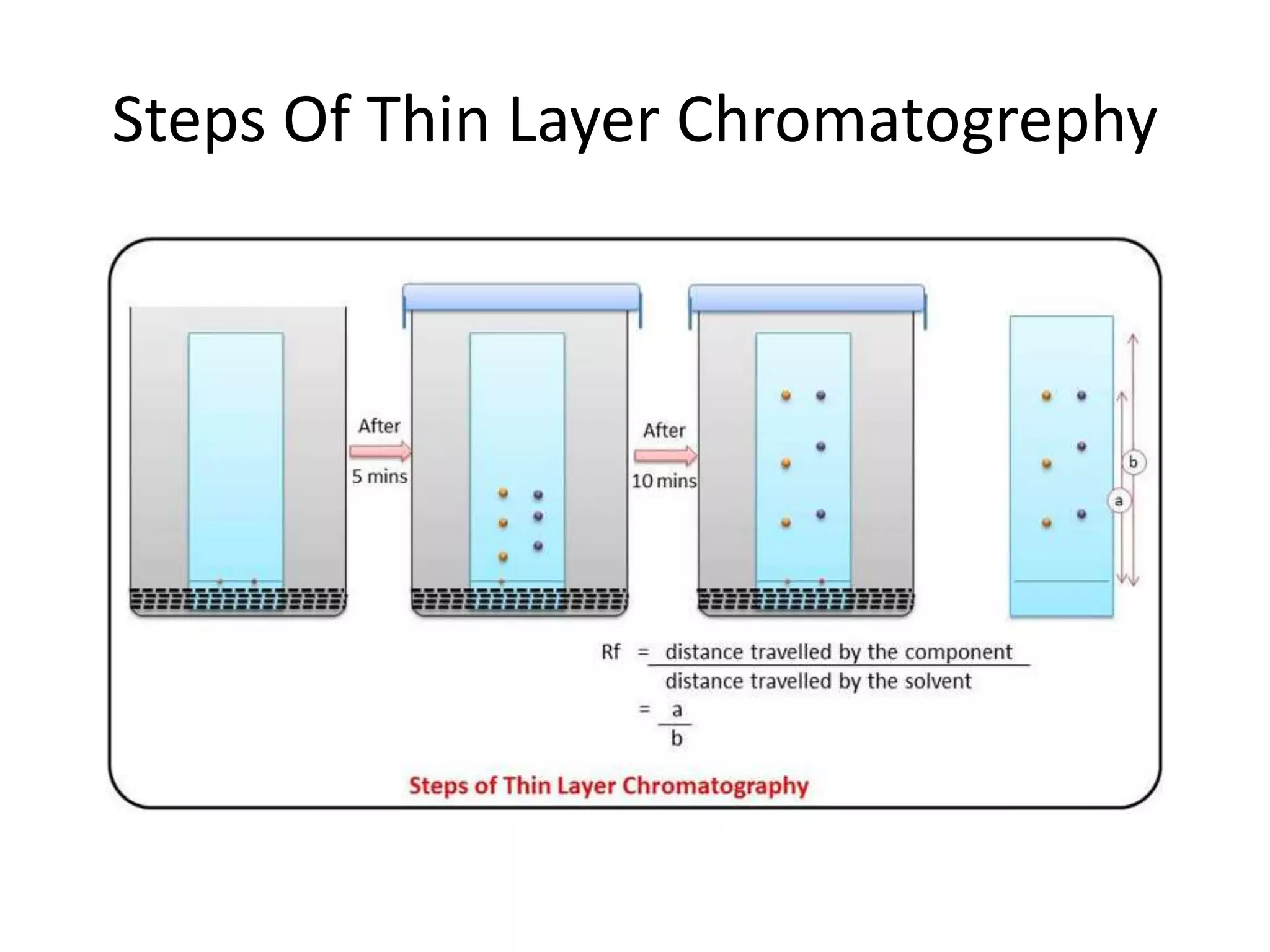
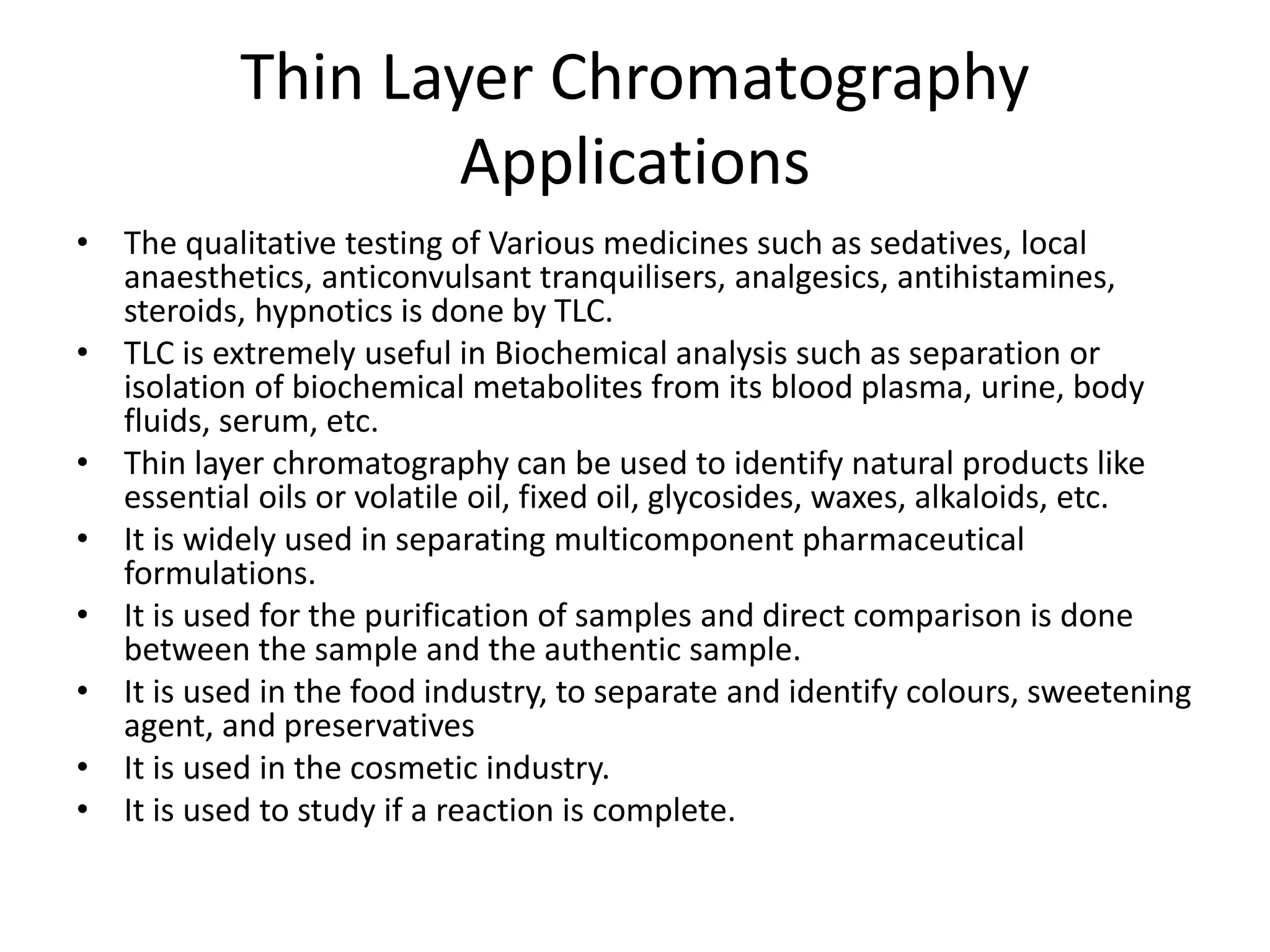
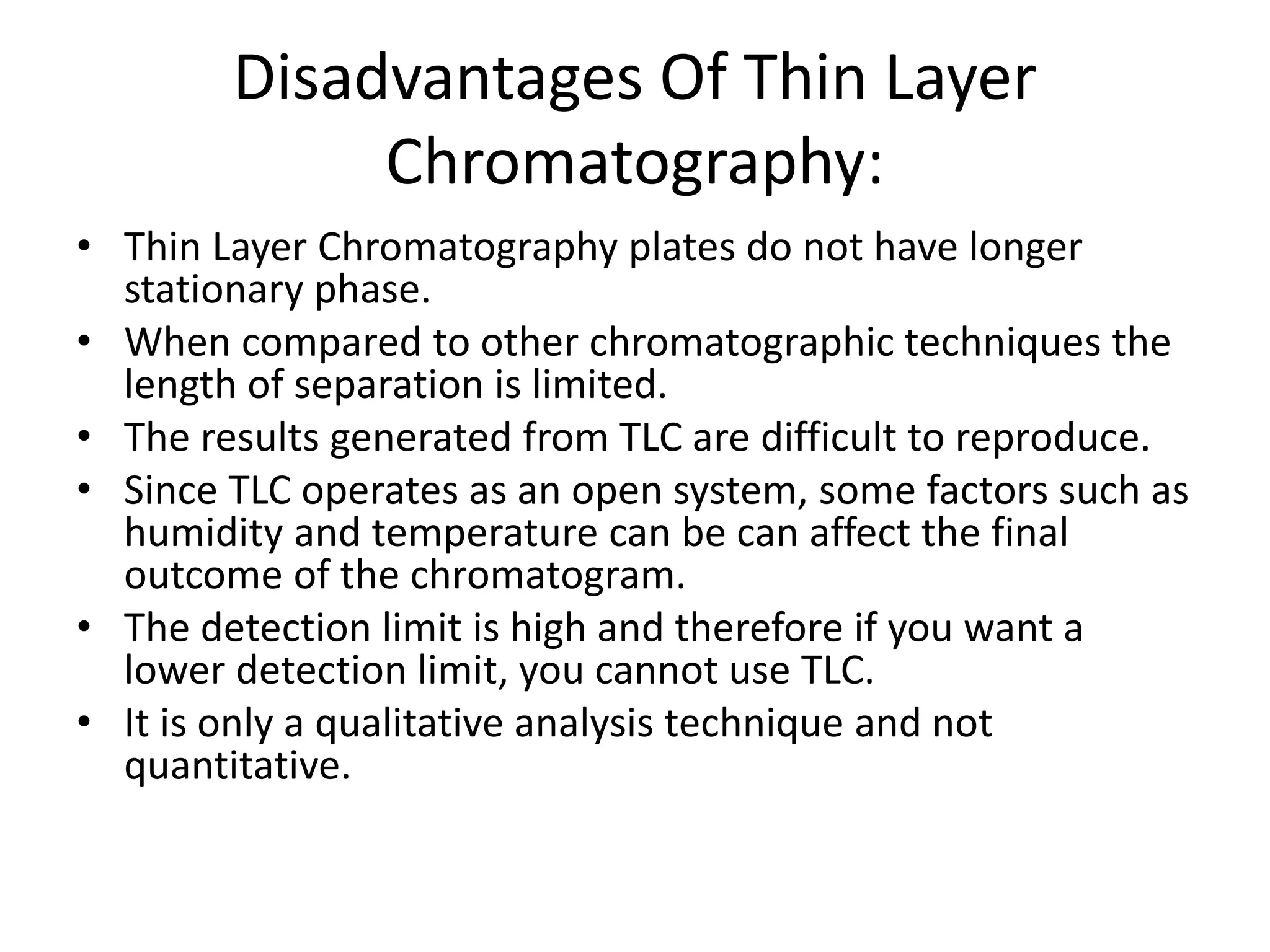
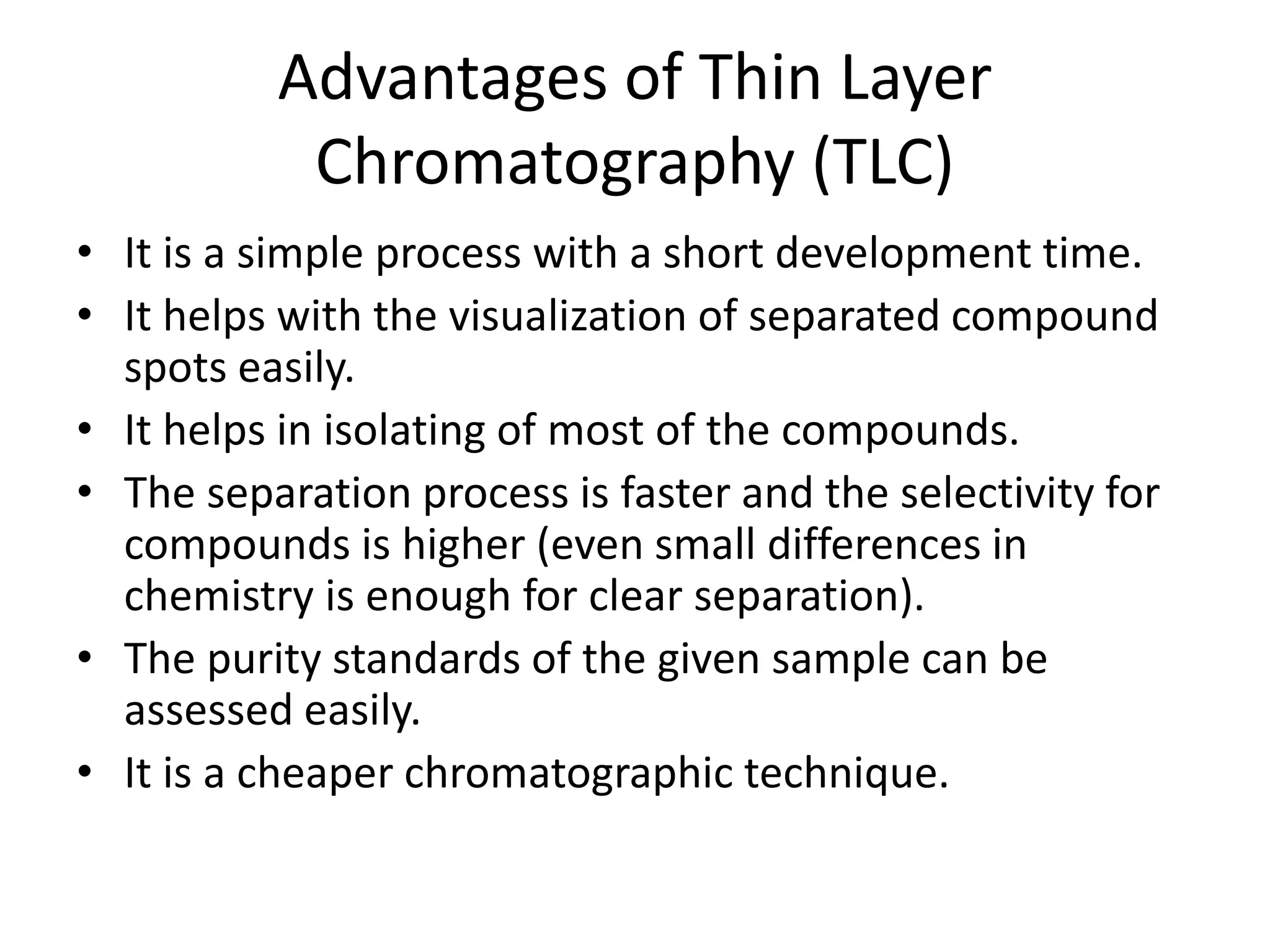
Thin layer chromatography is a technique used to separate mixtures. It works by using a stationary phase coated on a plate and a mobile phase that moves through the stationary phase. Samples applied to the plate separate into individual spots as they travel different distances up the plate based on their interactions with the phases. Factors like the solvent system, amount of material, adsorbent, and temperature affect the separation. TLC is a simple, fast, and inexpensive technique used in chemistry, biochemistry, medicine, forensics and other fields to separate and analyze mixtures.