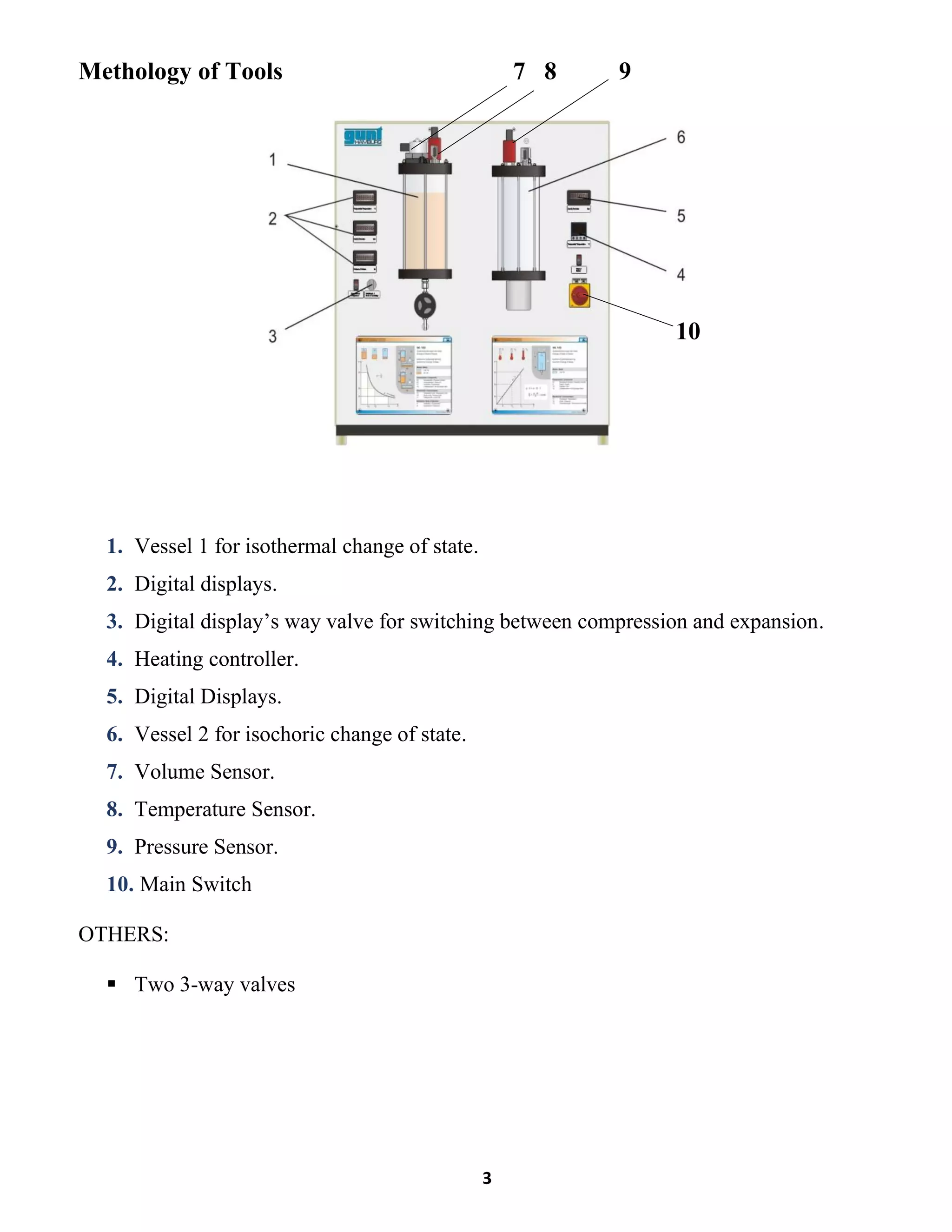
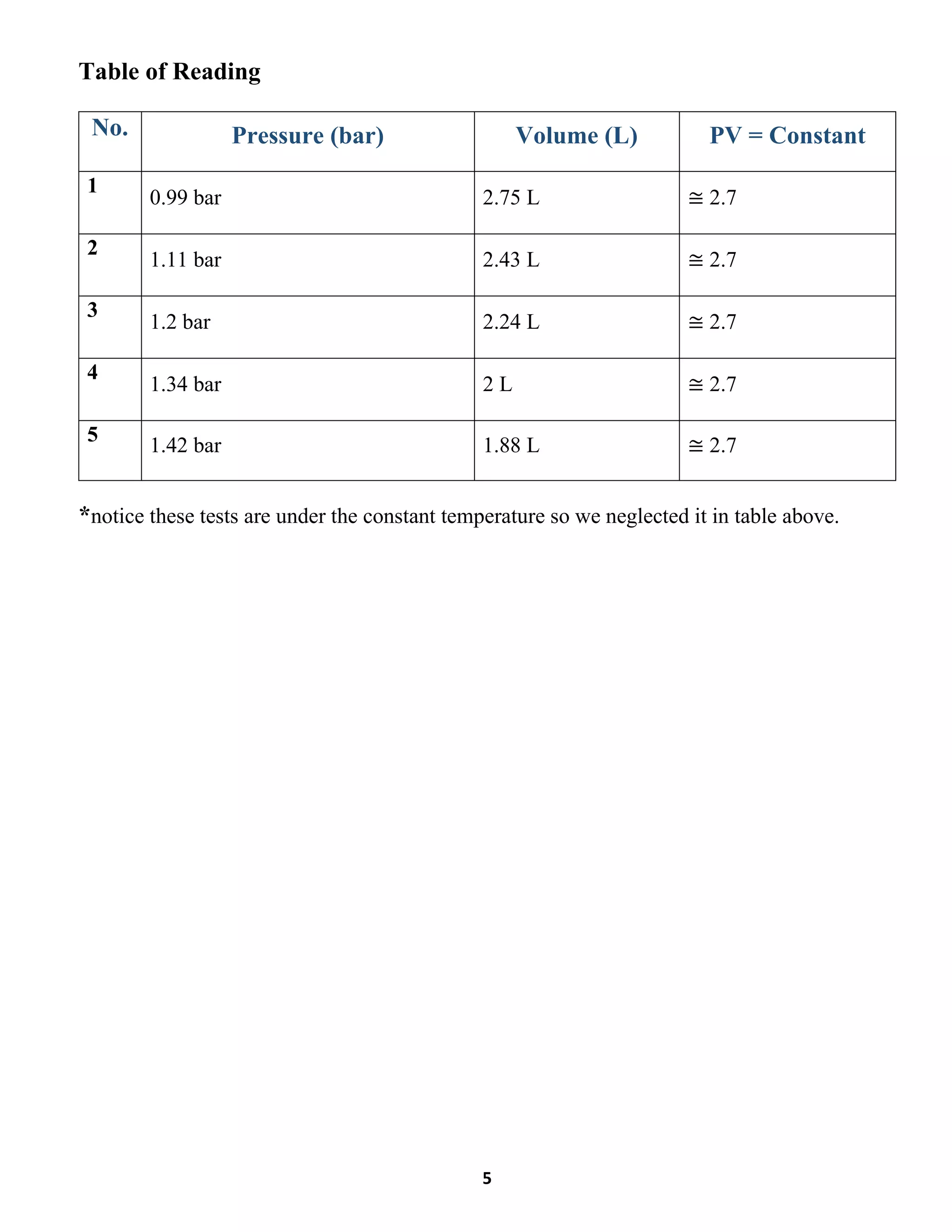
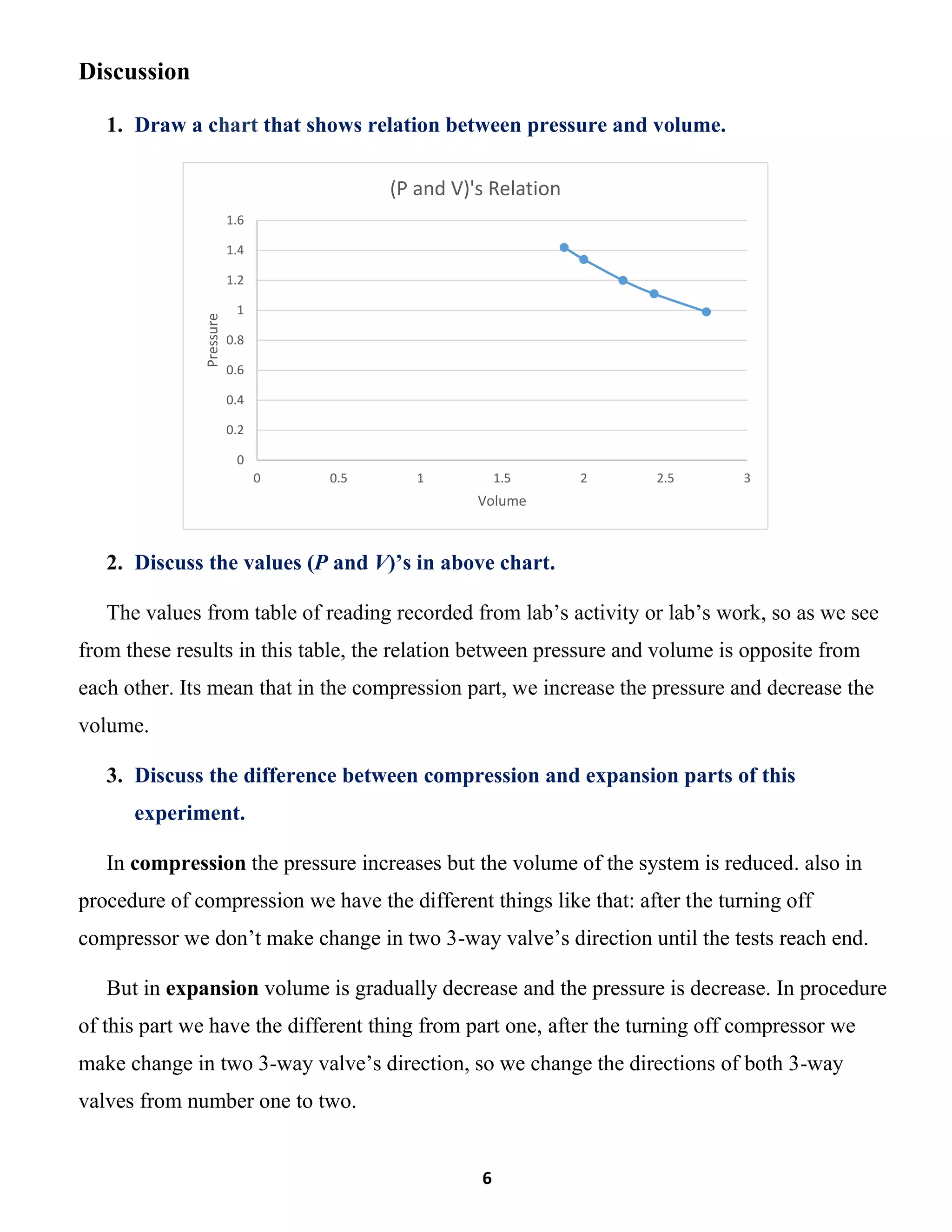
This lab report summarizes an experiment to verify Boyle's law regarding the inverse relationship between the pressure and volume of a gas at constant temperature. The experiment involved compressing a gas in a vessel and measuring the pressure and volume at various points. The results showed that as pressure increased, volume decreased, maintaining a constant product of pressure and volume. Key differences between the compression and expansion parts of the experiment were discussed.