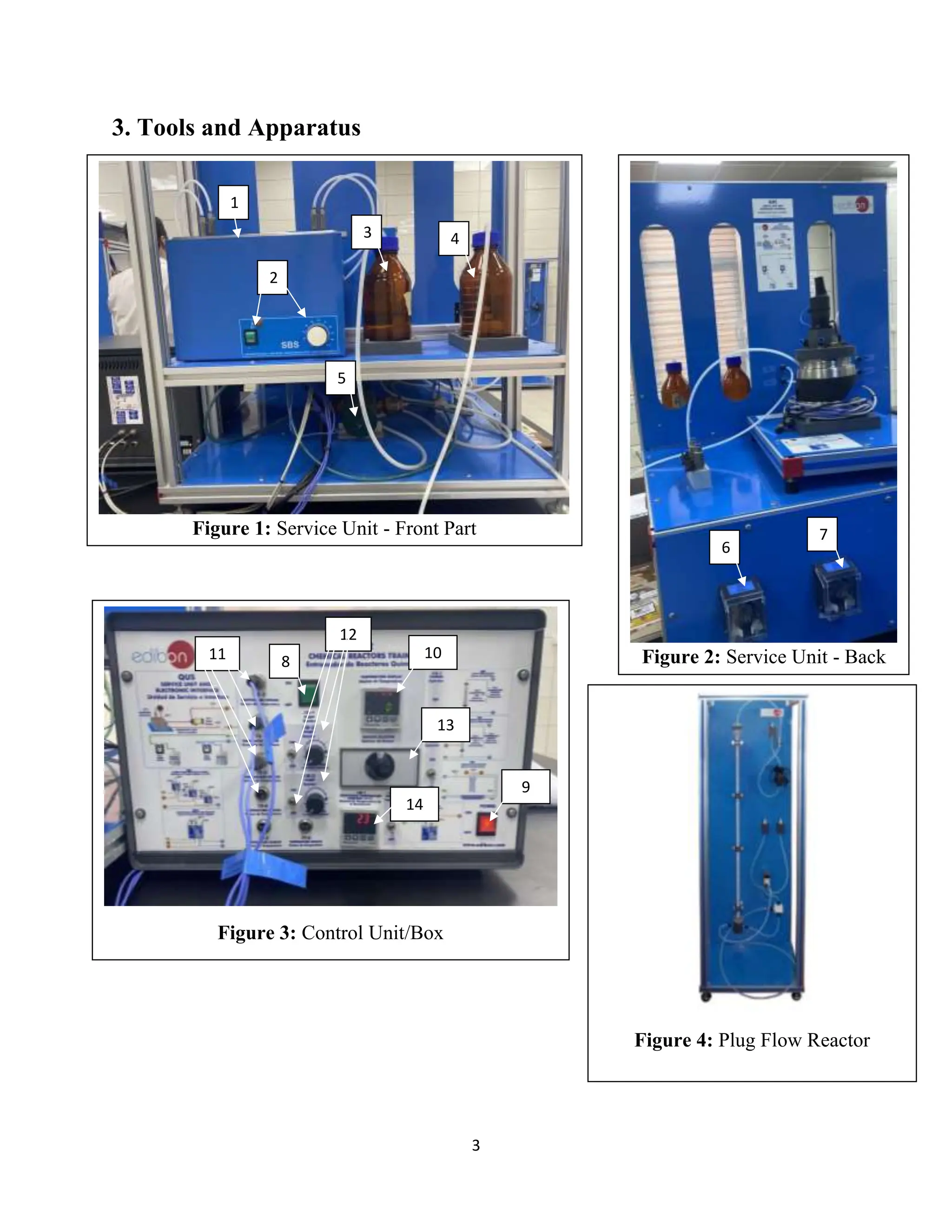
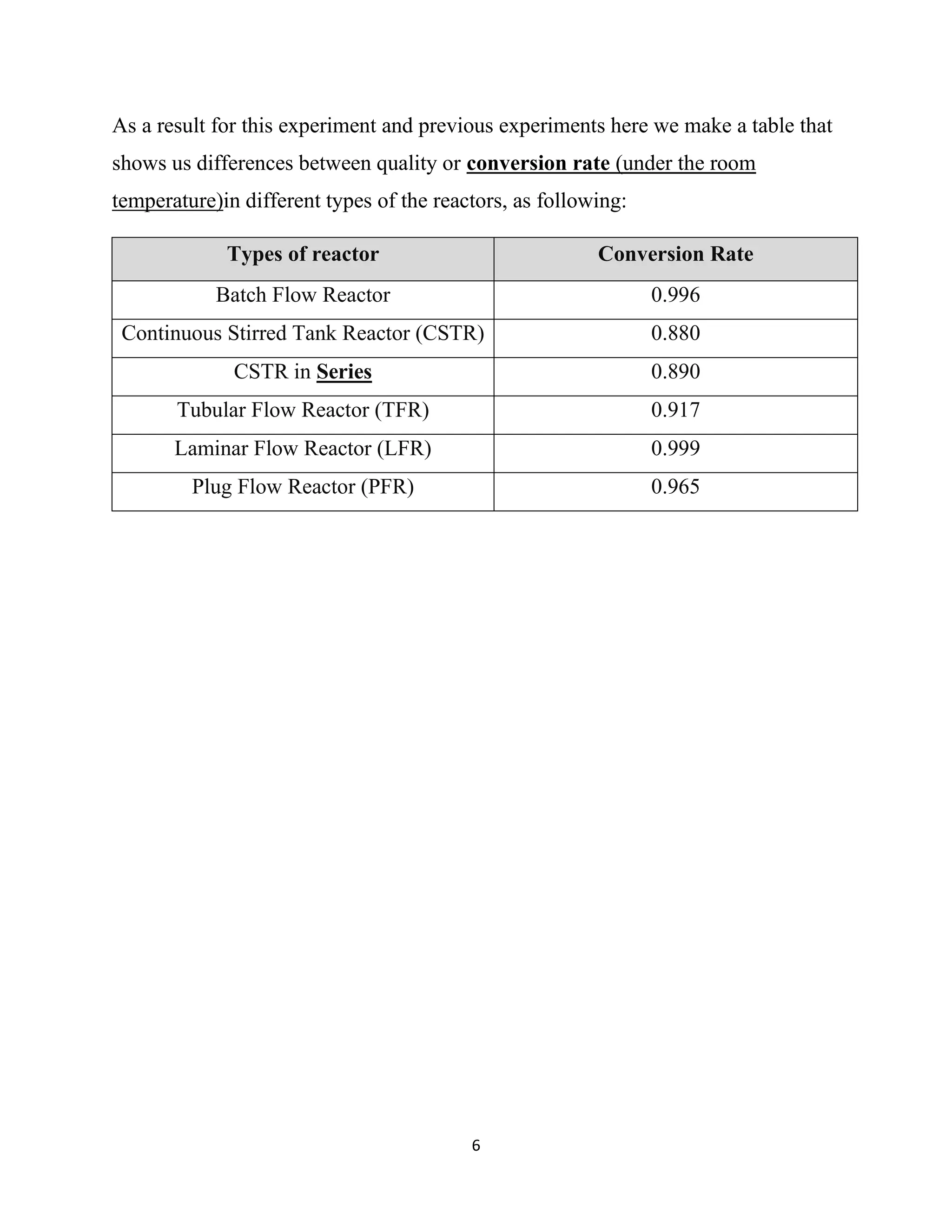
The document describes a lab experiment conducted to determine the conversion of a plug flow reactor (PFR) at 20°C. Two reactants, 0.05M NaOH and 0.05M CH3COOHC2H5, were pumped through the PFR and the conductivity was measured to calculate conversion. The conversion was found to be 0.965. Previous experiments showed laminar flow reactors have the highest conversion rates, followed by batch reactors and then PFRs. Group members discussed the procedure, calculations, and comparison to other reactor types.