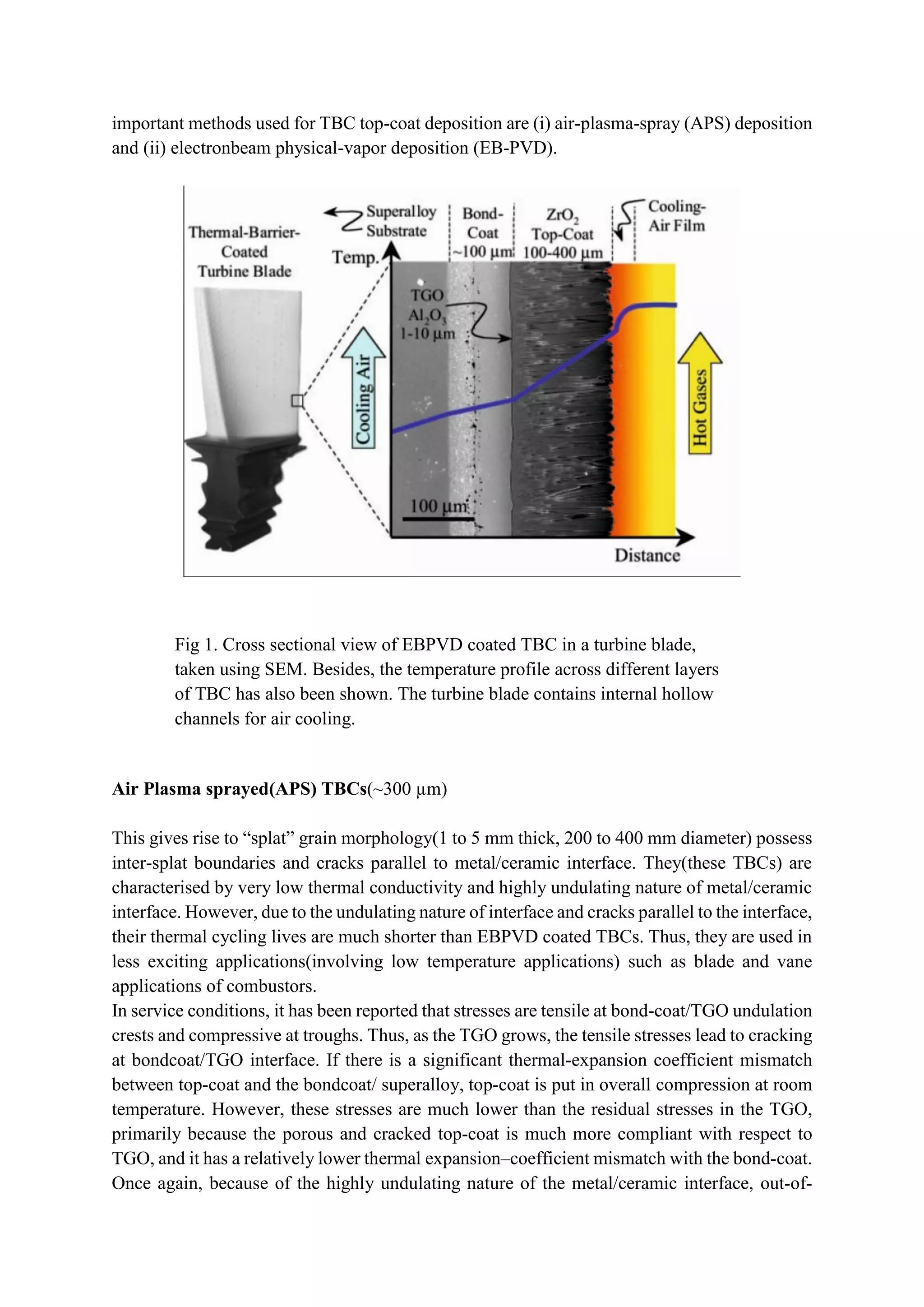
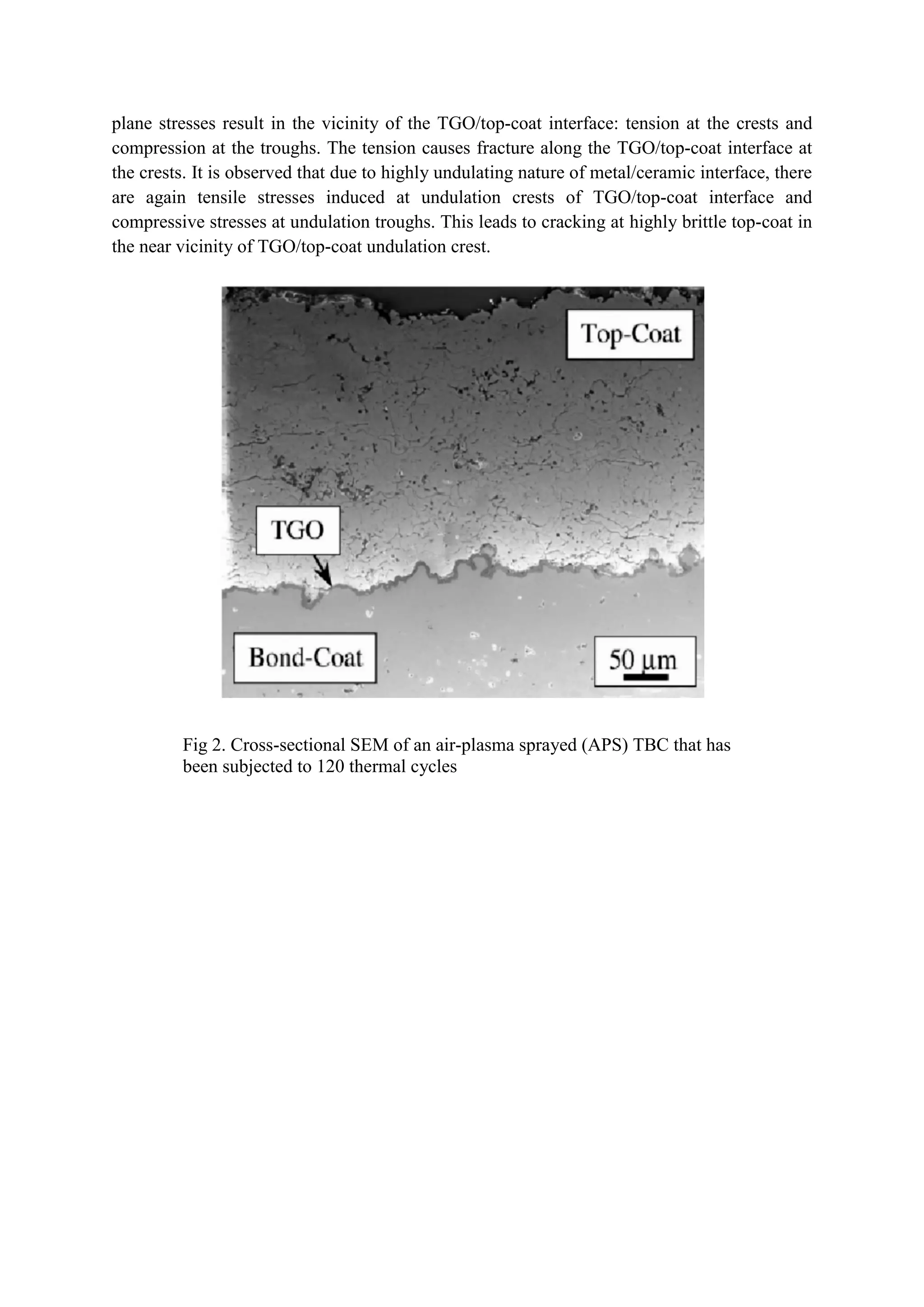
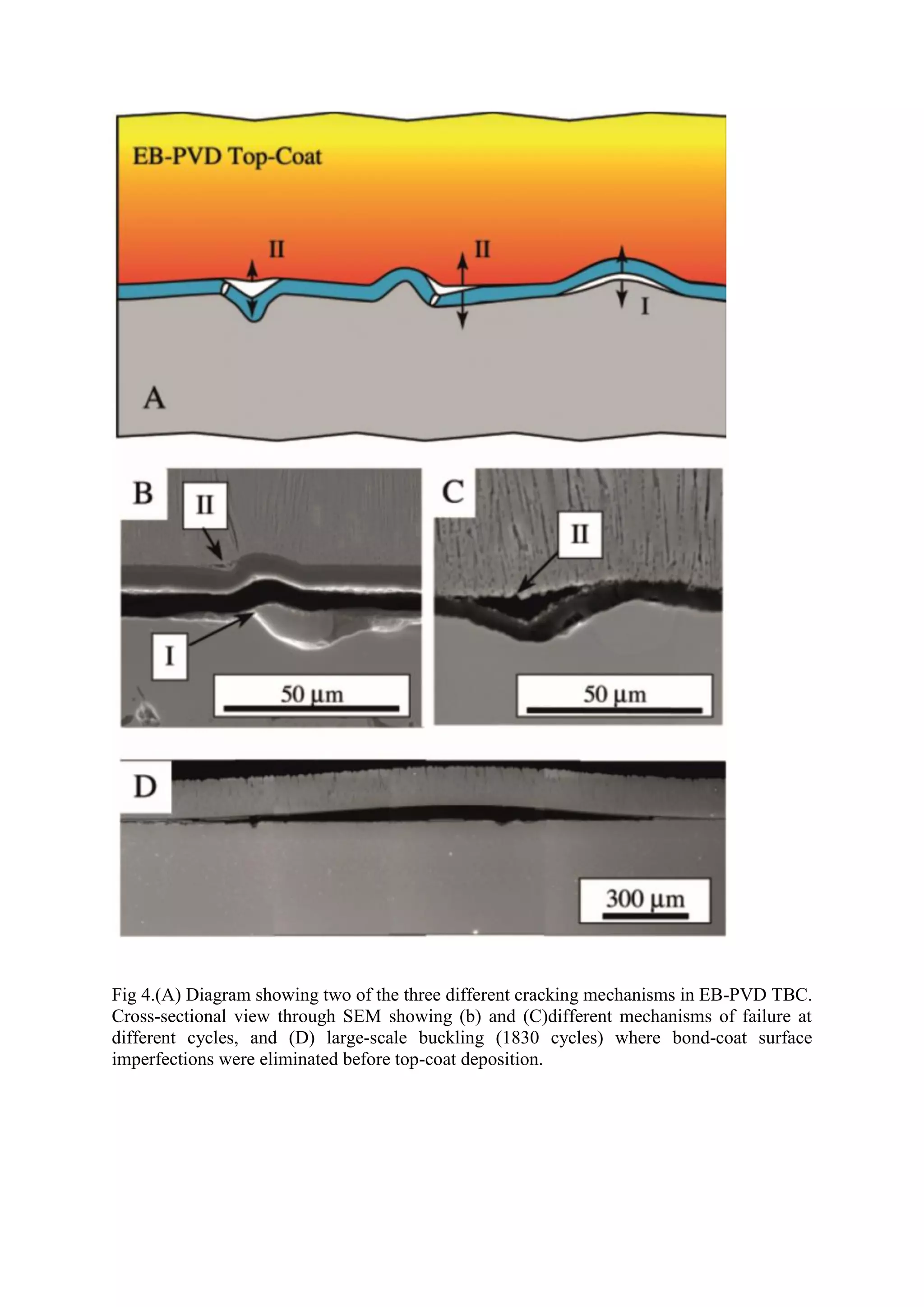
Thermal Barrier Coatings (TBCs) are coatings applied to gas turbine engine components to increase their high-temperature capability. TBCs typically have four layers: a superalloy substrate, bond coat, thermally grown oxide layer, and yttria-stabilized zirconia ceramic topcoat. TBCs allow gas turbine blades to operate at temperatures up to 1500°C, significantly increasing engine efficiency. However, TBCs can fail over time due to thermal expansion mismatches and oxidation of the bond coat, reducing the operating life of coated components.