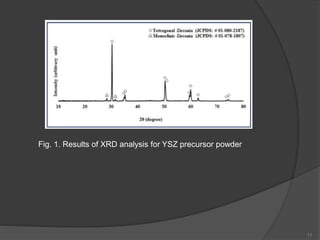
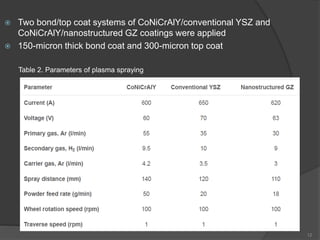
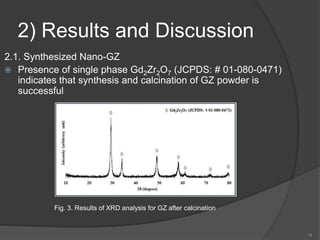
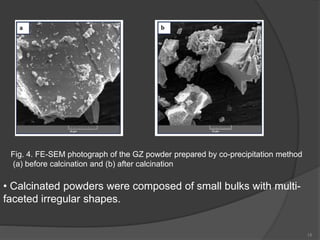
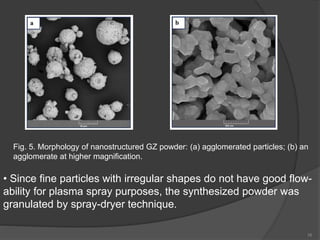
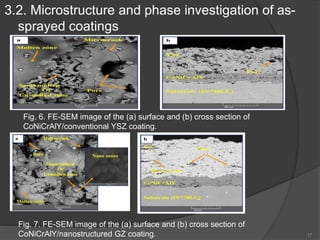
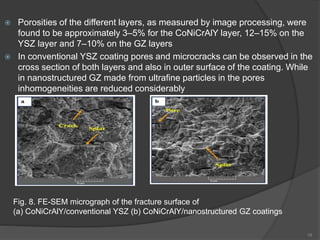
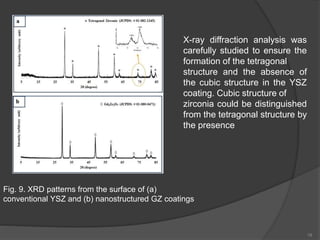
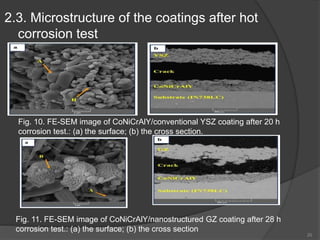
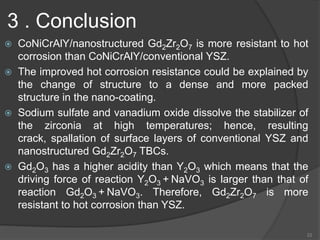
The document discusses thermal barrier coatings (TBC) used to insulate metallic parts from high temperatures, thus enhancing engine performance. It details the structure, material selection, and various deposition methods alongside a case study that emphasizes the advantages of nanostructured Gd2Zr2O7 coatings over conventional YSZ in terms of hot corrosion resistance. The findings indicate that the nanostructured coatings present improved structural integrity and thermal stability, making them more effective in high-temperature environments.