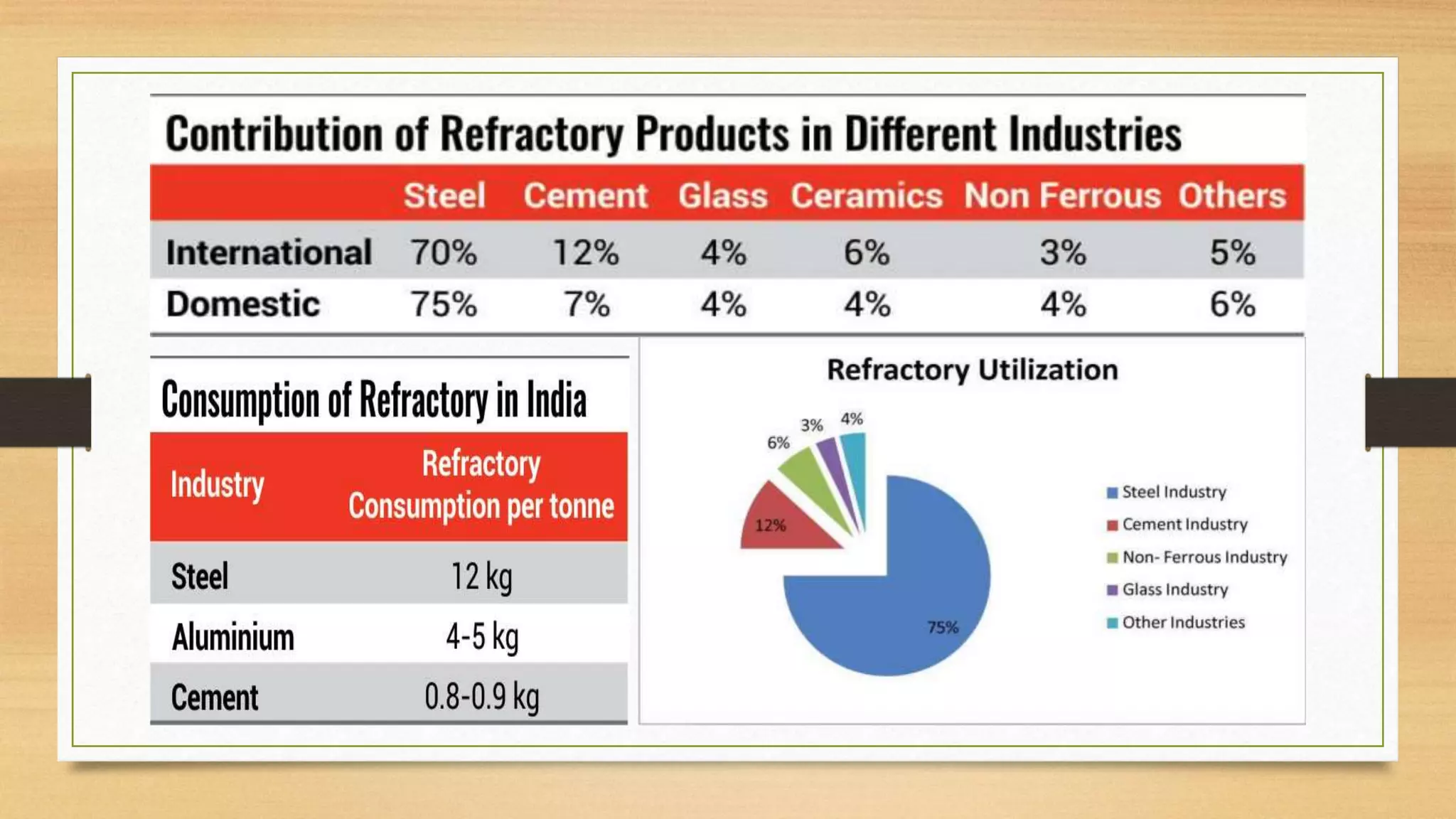
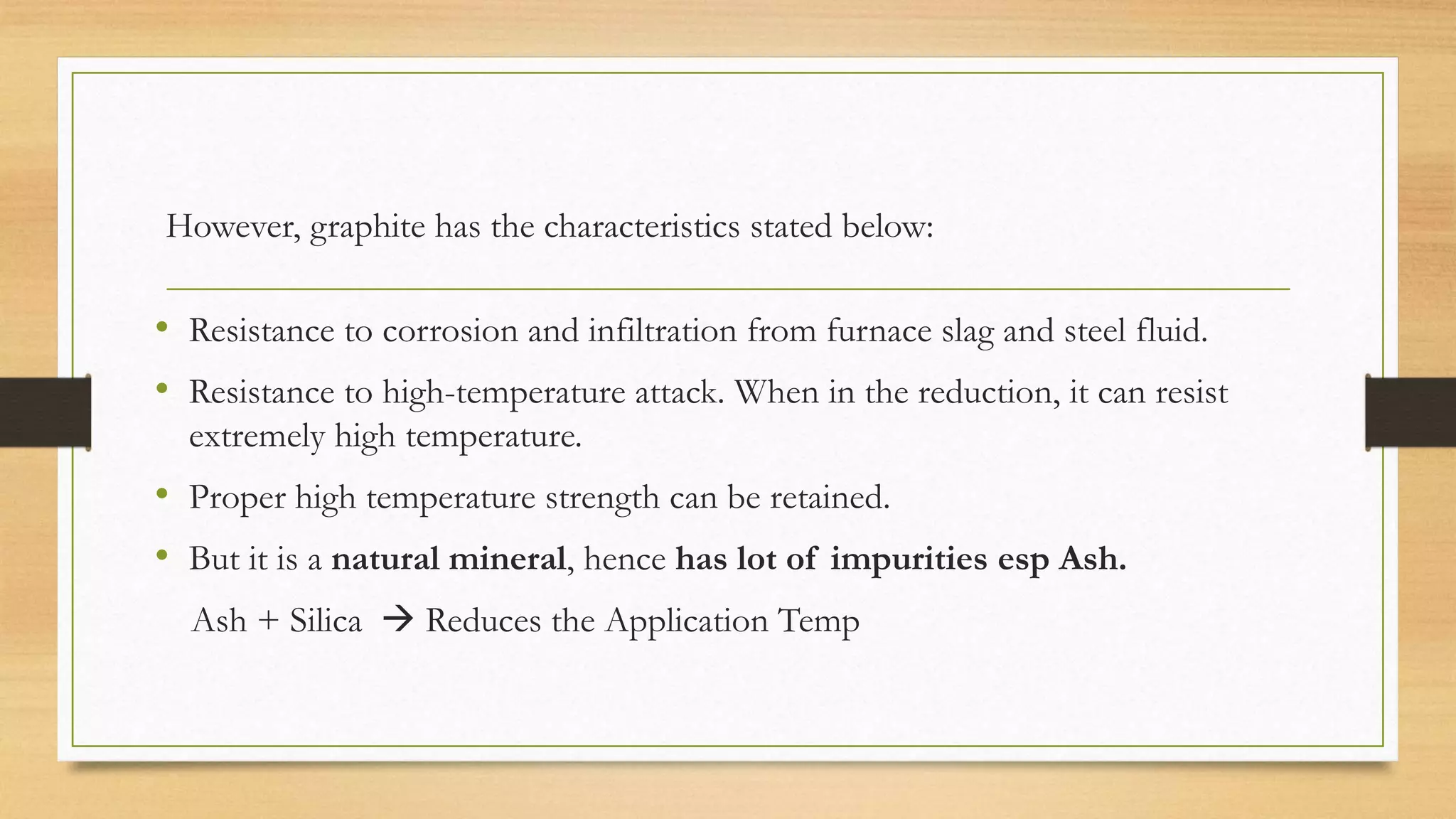
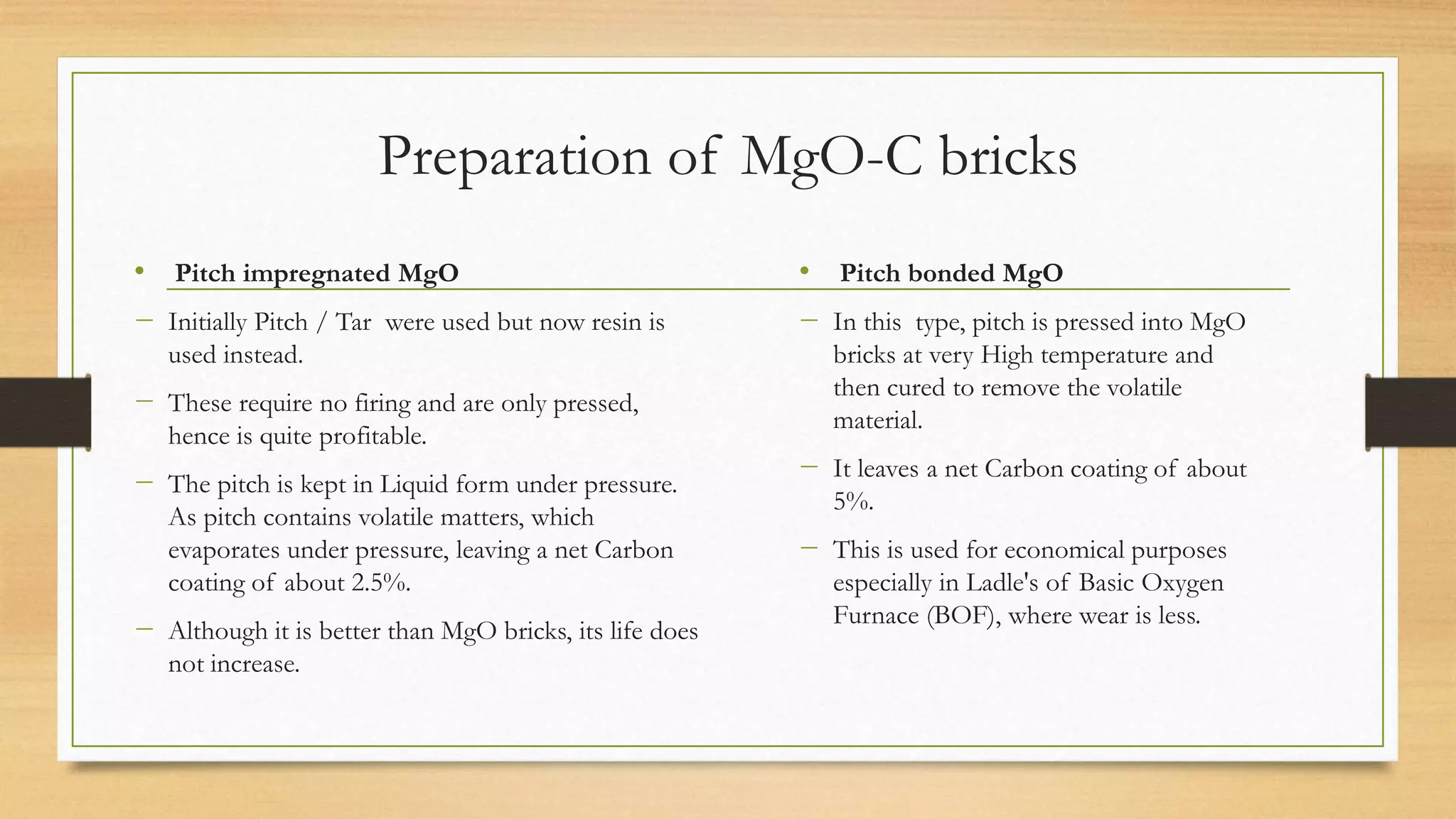
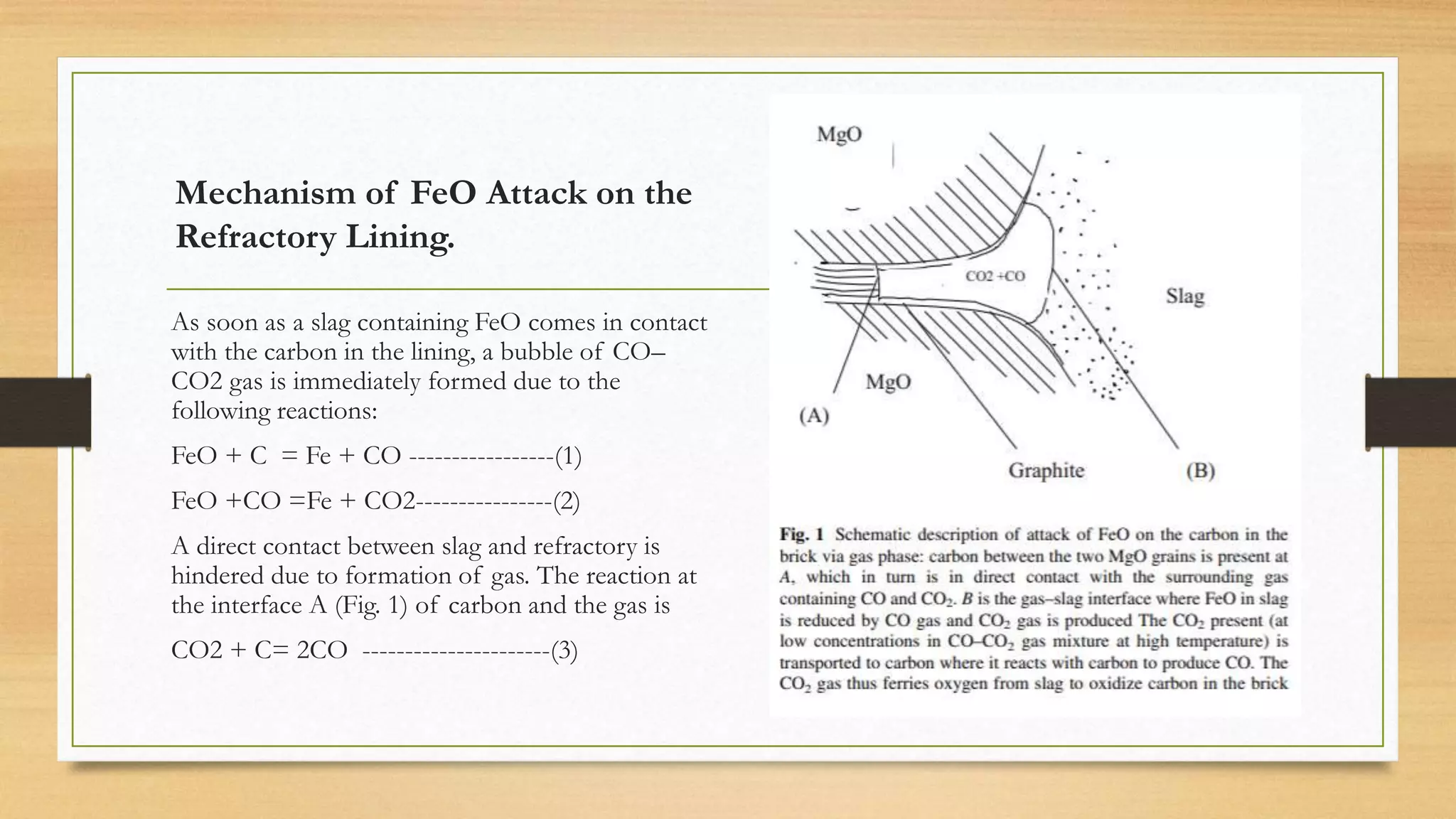
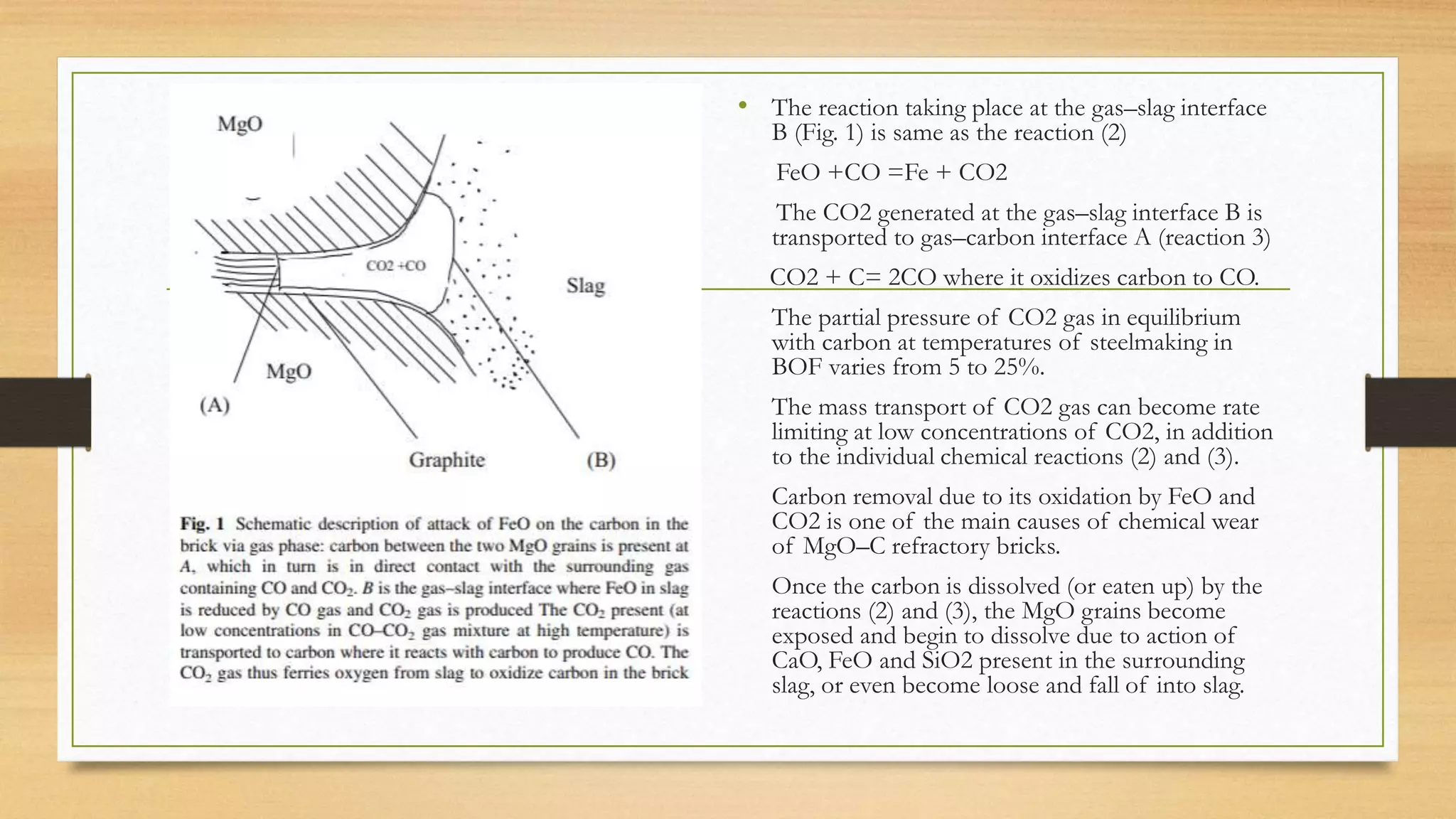
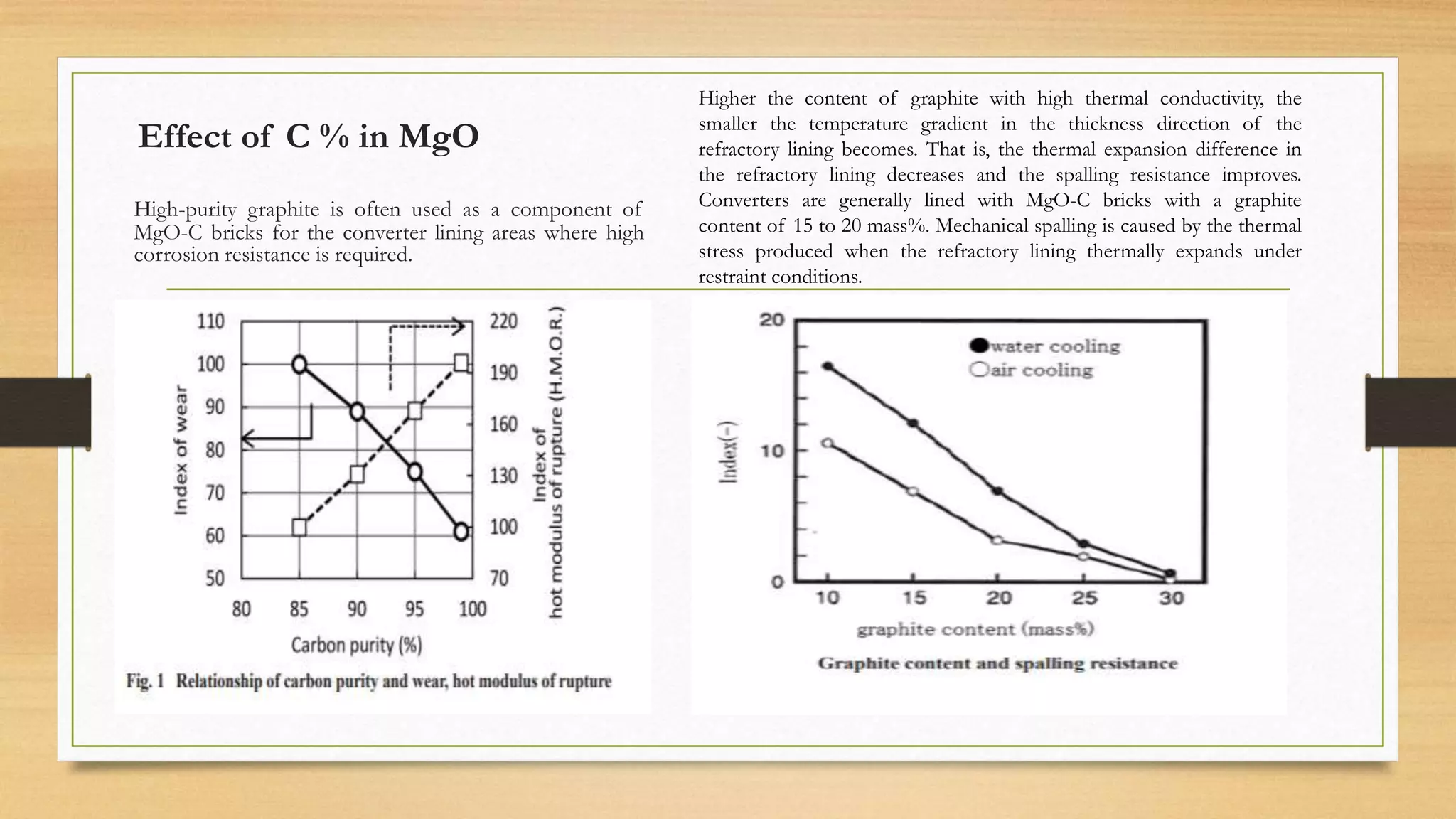
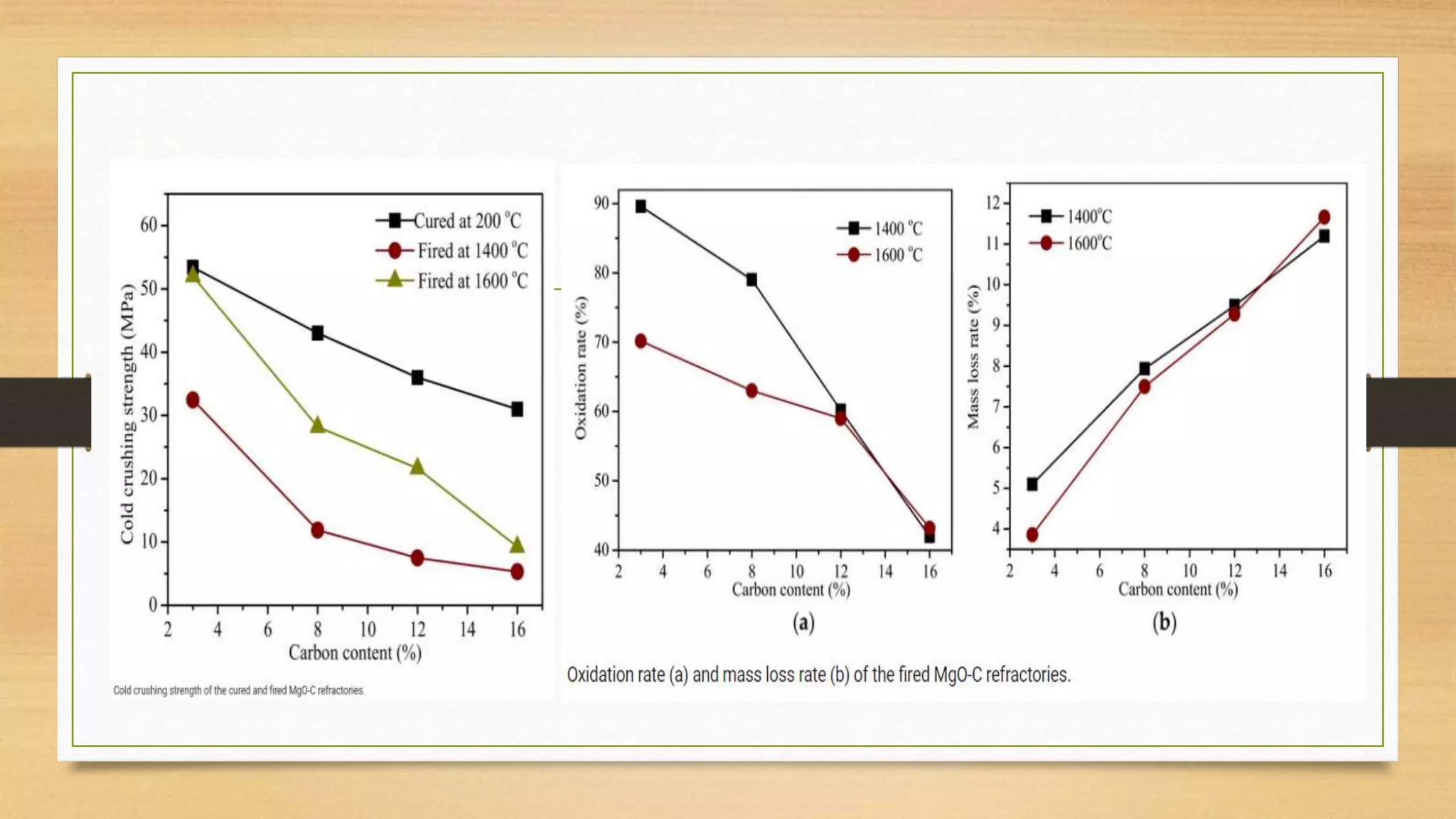
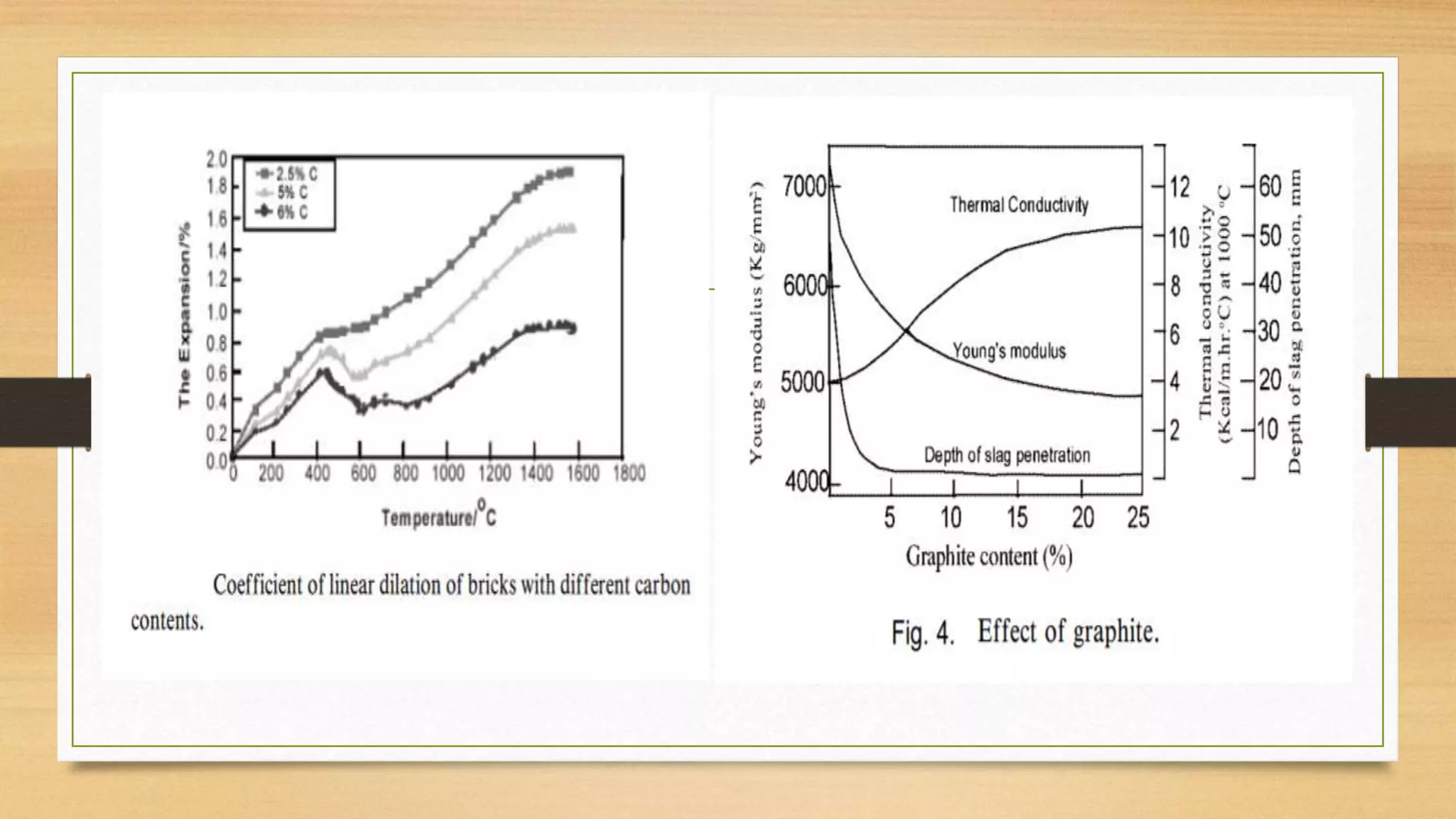
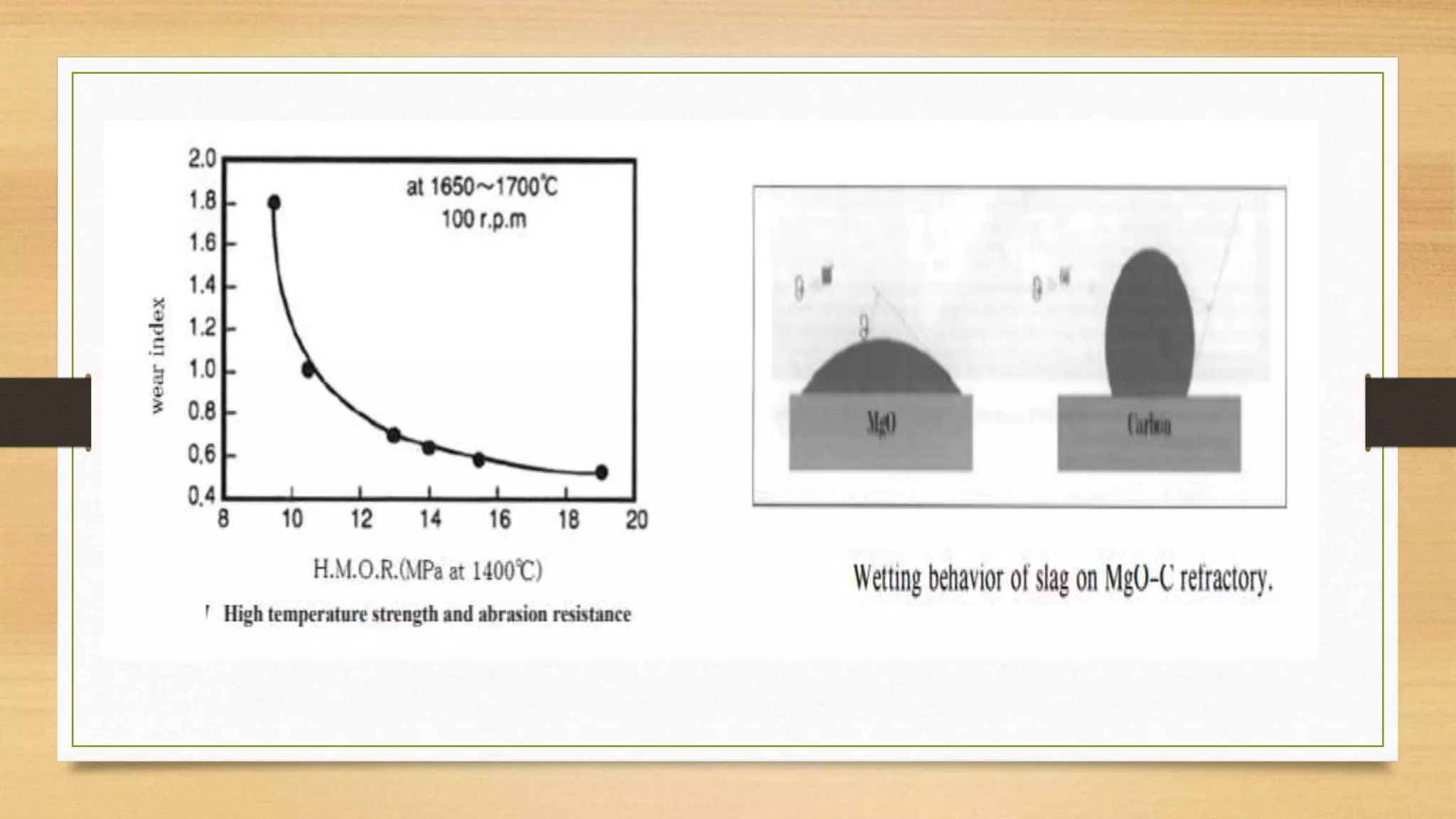
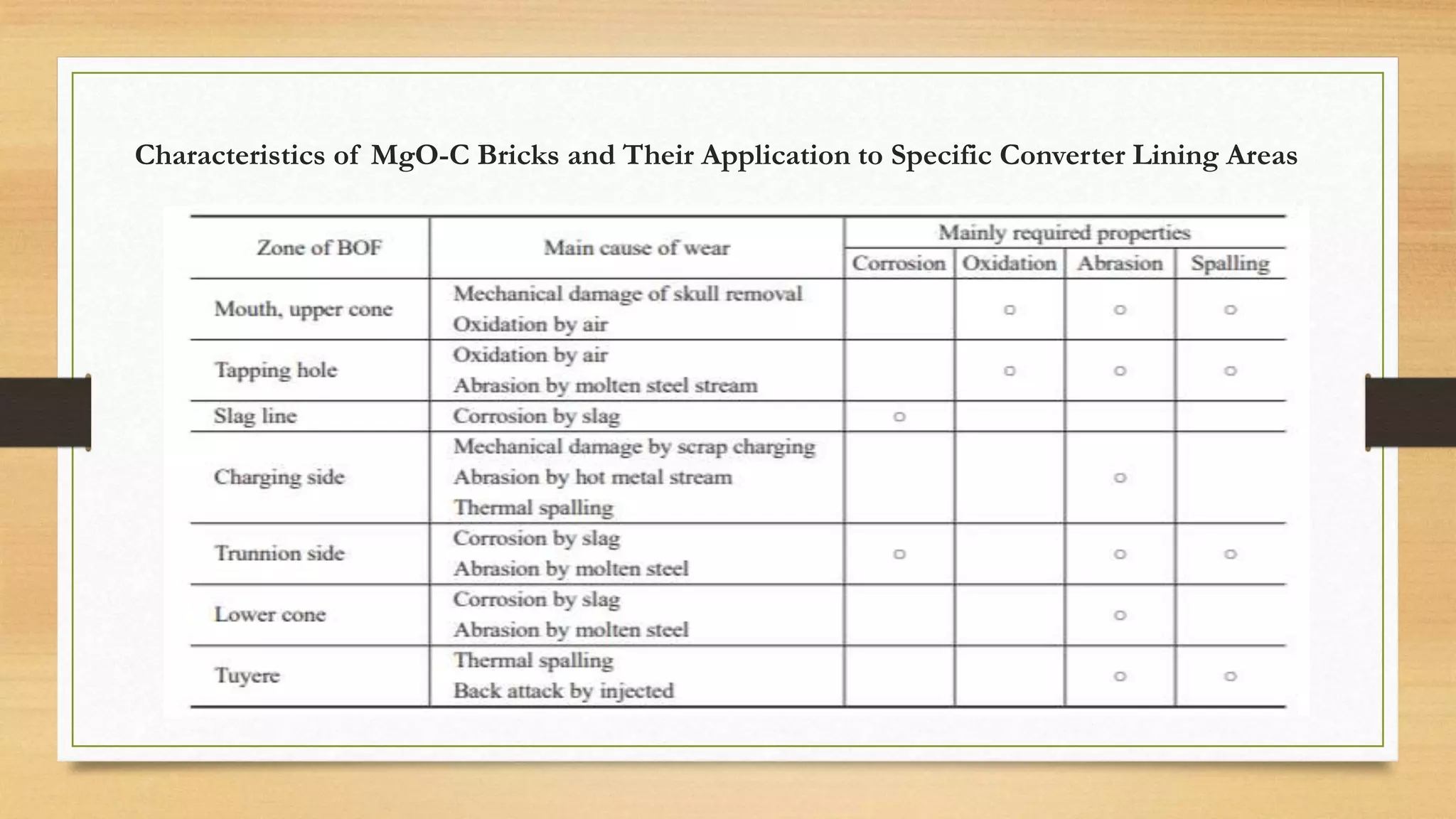
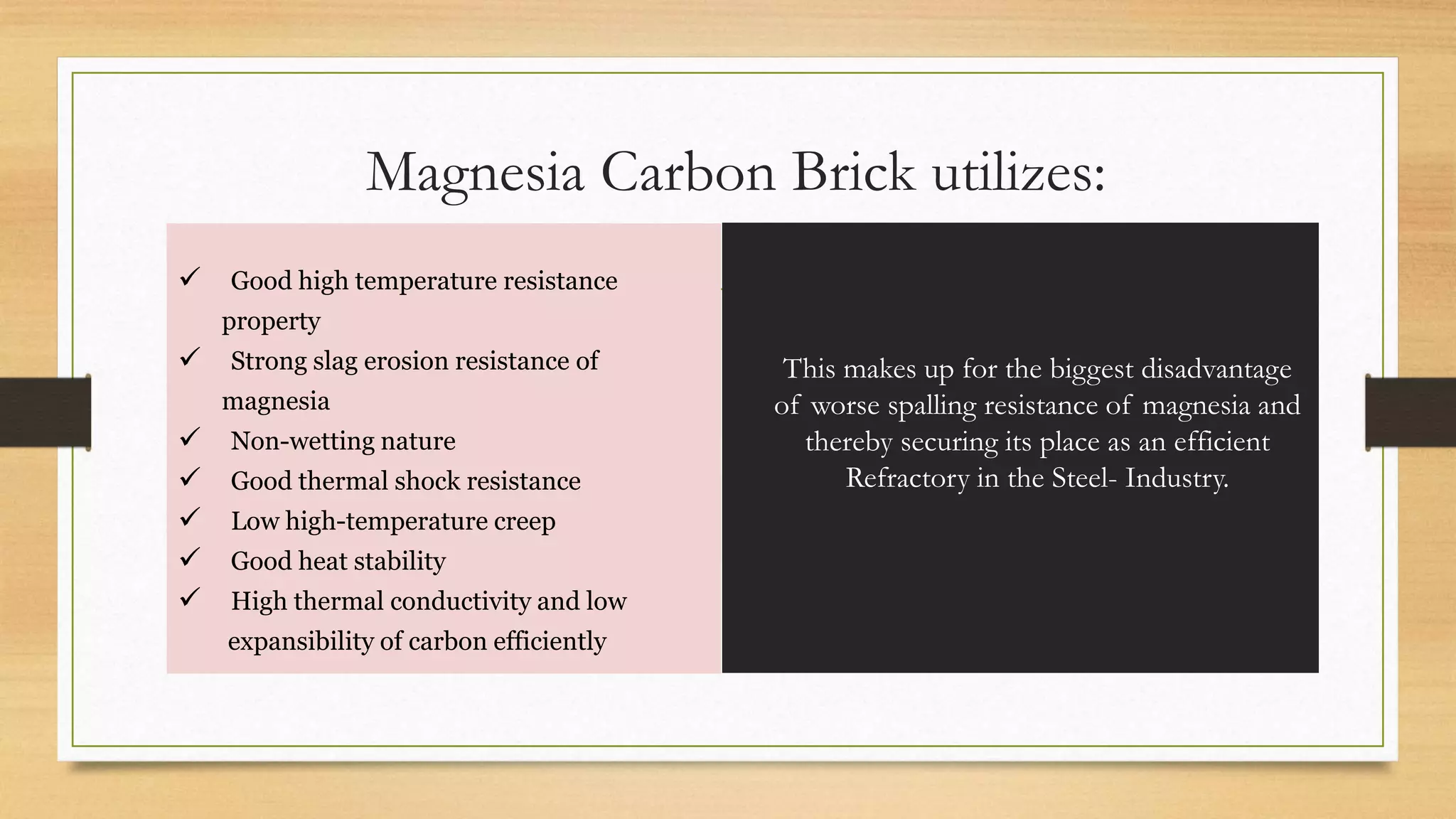
This document summarizes information about refractory-magnesia carbon bricks and their use in the steel industry. It discusses that magnesia-carbon bricks are a non-fired basic refractory material used as a lining in basic oxygen furnaces. The bricks contain magnesia for its refractory properties and graphite for its corrosion and thermal conductivity properties. The document outlines the preparation process for magnesia-carbon bricks and explains the mechanisms of how iron oxide in slag can react with and dissolve the carbon component over time, exposing the magnesia grains to further corrosion. It also discusses how varying the graphite content can affect the thermal properties and spalling resistance of the refractory lining.