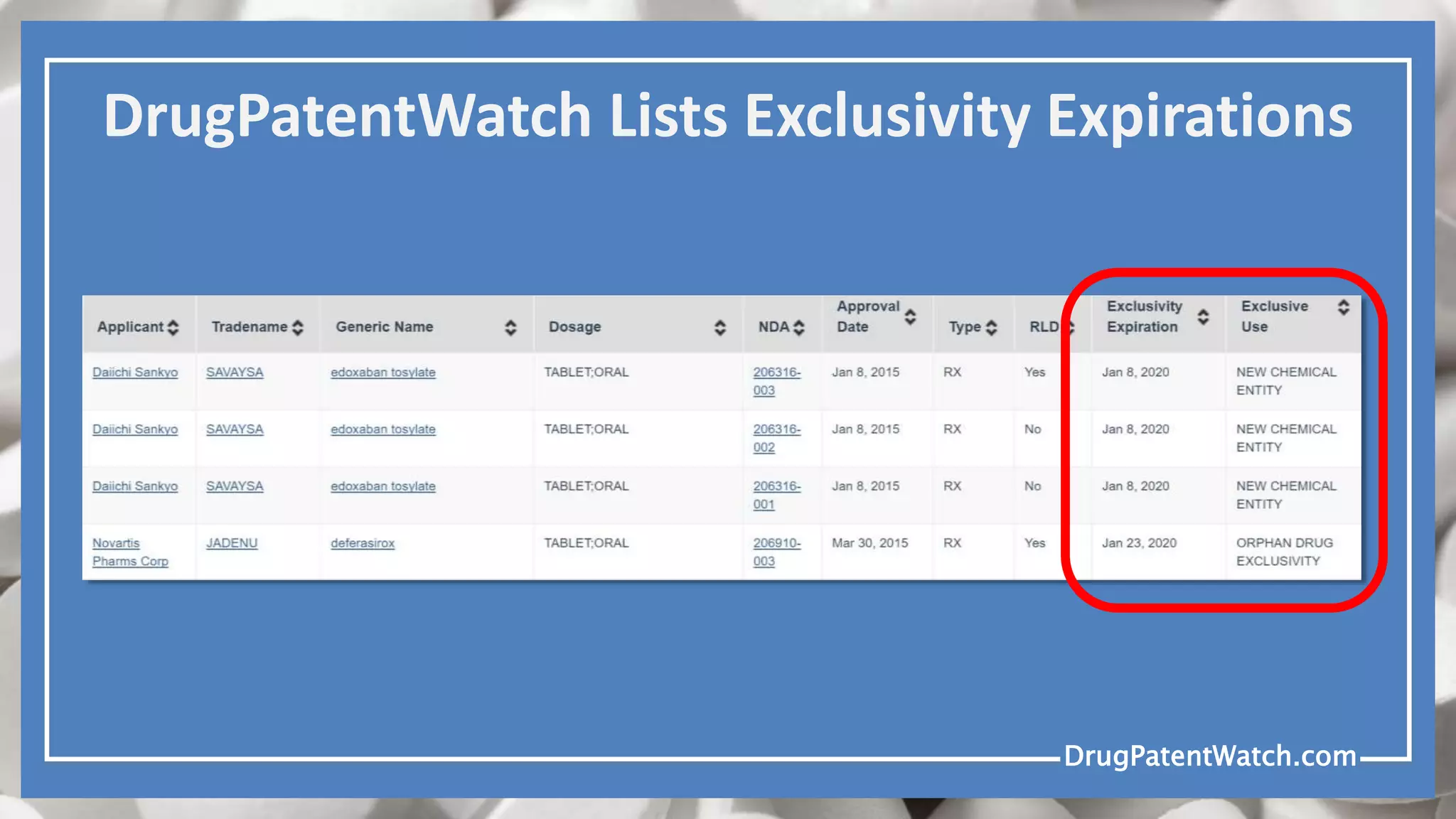
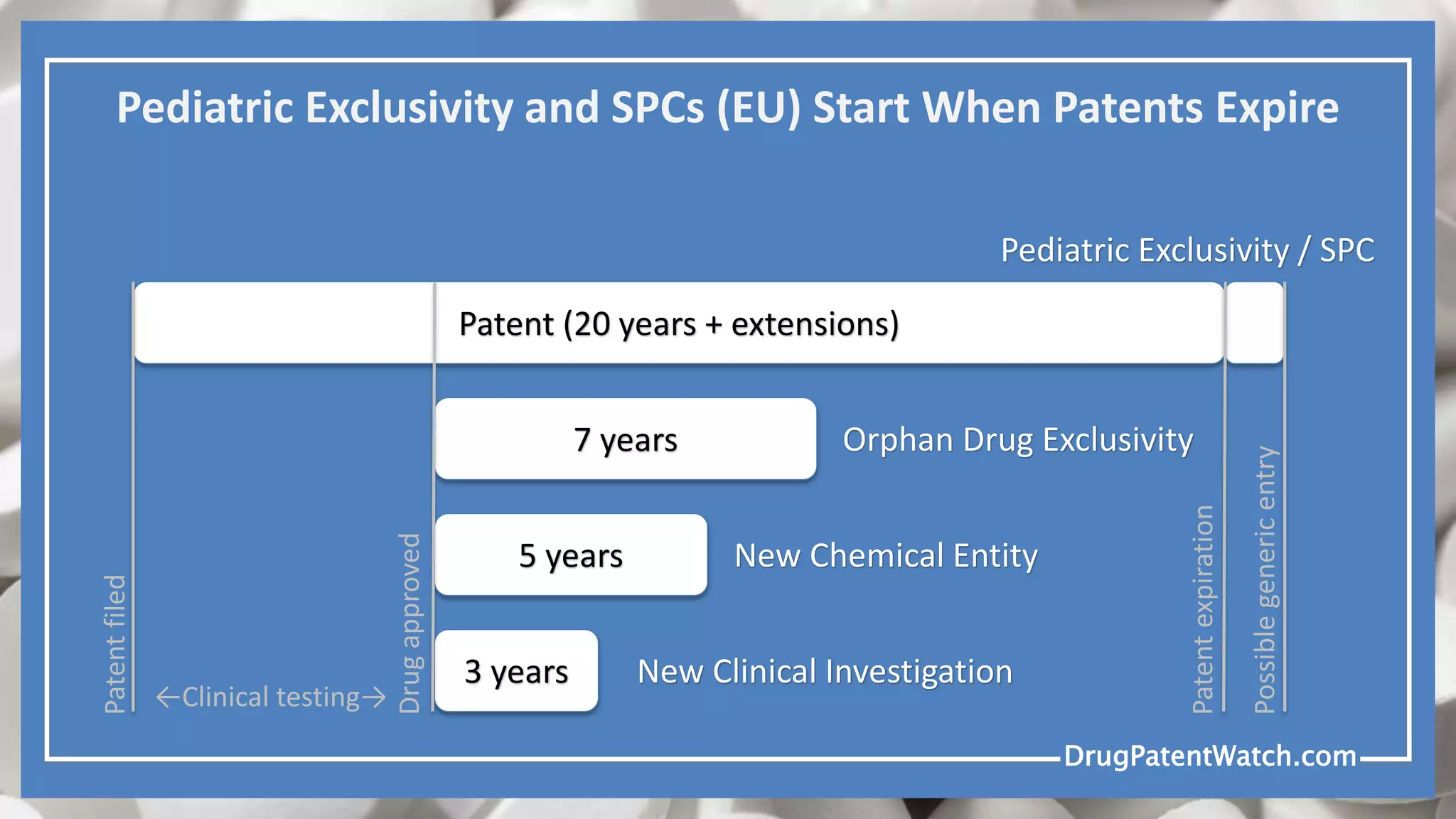
Drug patents typically expire 20 years from the filing date, but extensions and regulatory exclusivities can complicate this timeline. Generic drugs cannot be launched until all blocking patents and regulatory exclusivities have expired. Various types of patent claims may allow for generic entry under specific circumstances, such as different production methods or unpatented uses.