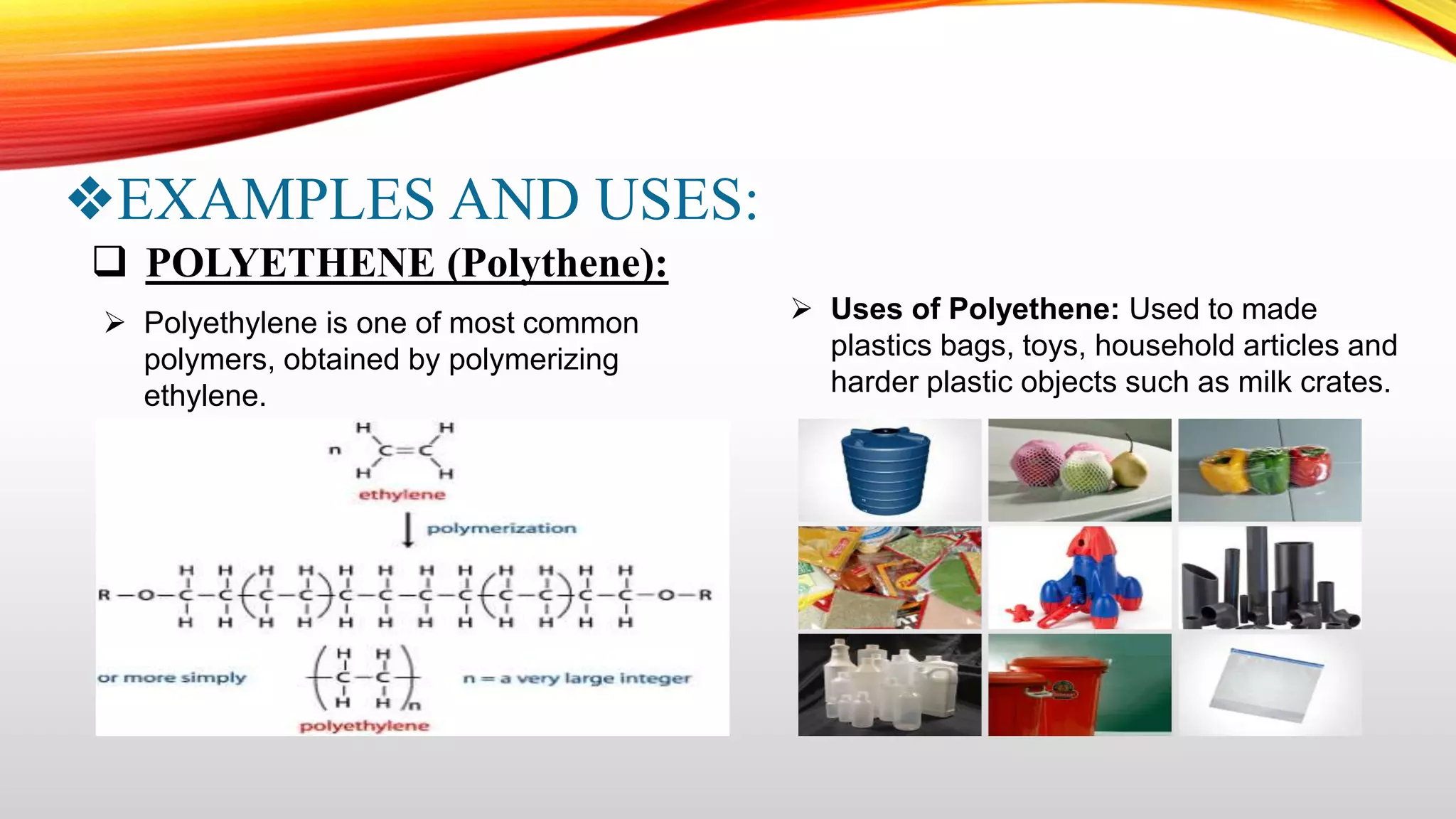
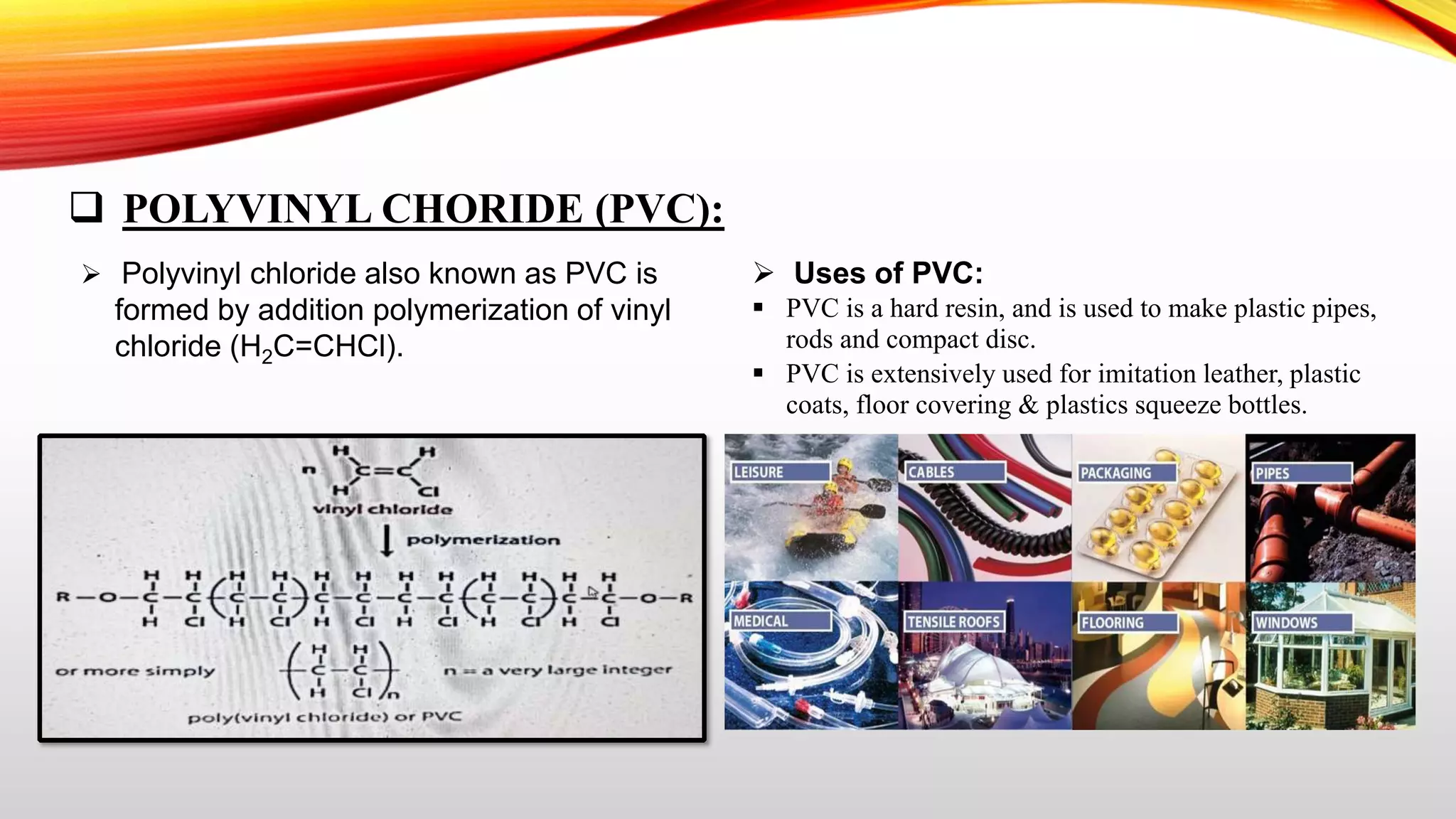
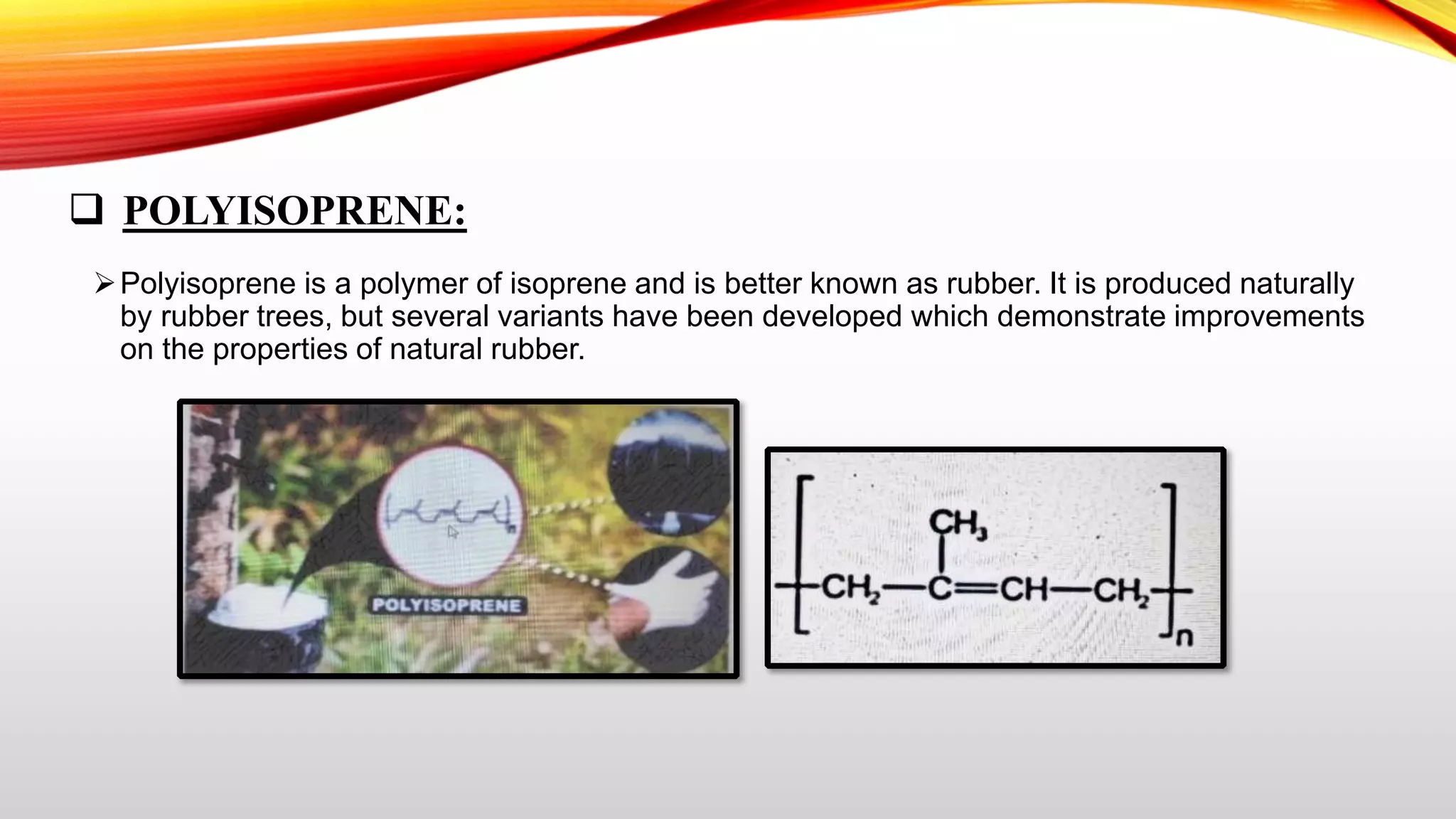
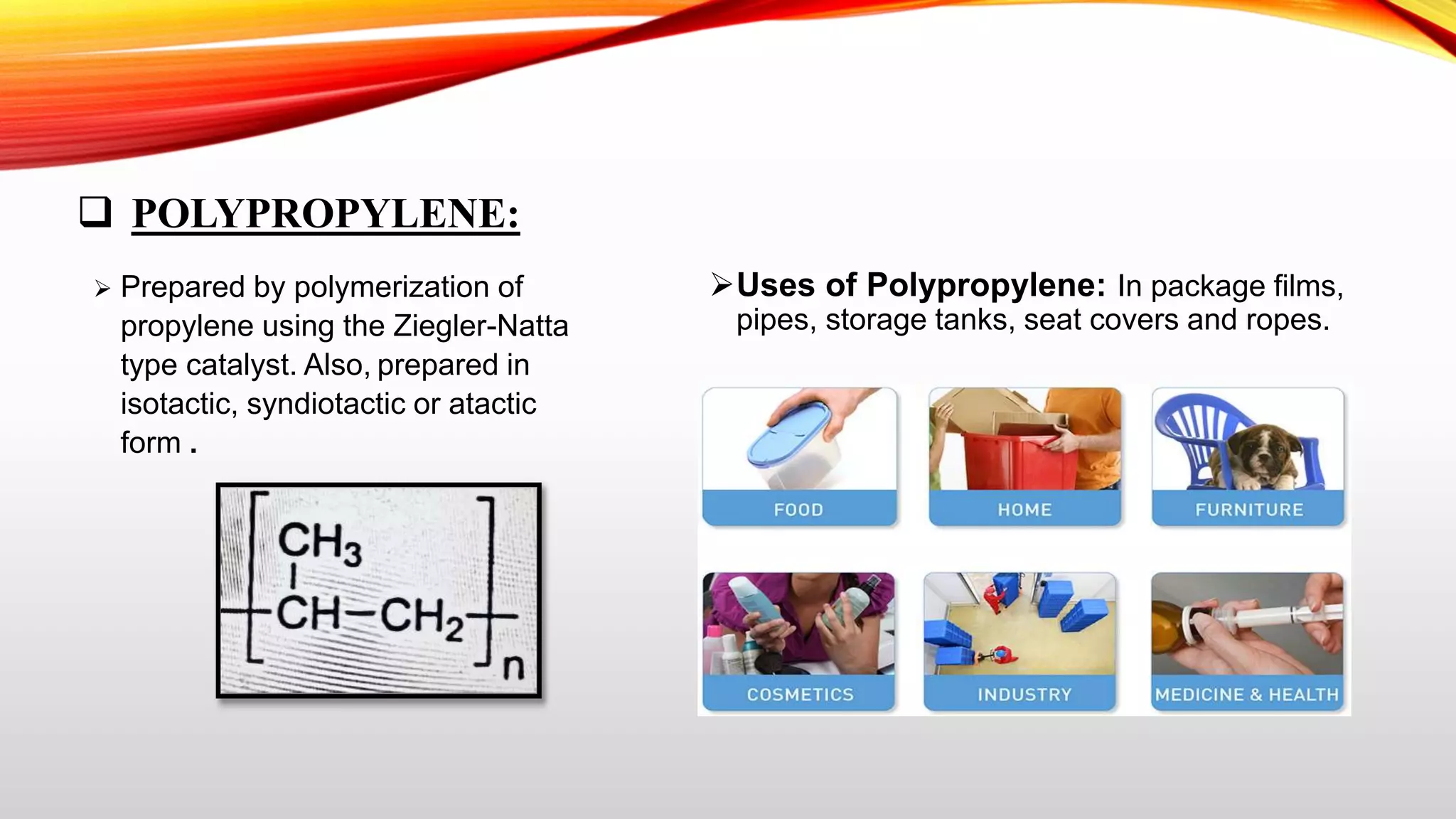
This document summarizes addition polymerization and provides examples. Addition polymerization involves a chain reaction where monomers add together without eliminating any byproducts to form long polymer chains. Examples discussed include polyethylene, used to make plastic bags and toys, polyvinyl chloride used for pipes and imitation leather, polyisoprene which is natural rubber, and polypropylene used for food packaging and other applications. The project aims to study addition polymerization and how it is used to form common plastic materials through a repetitive addition reaction of monomers.