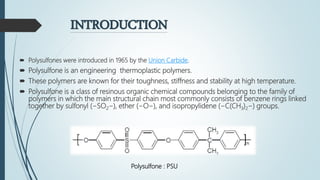
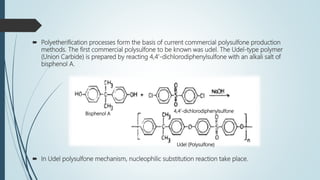
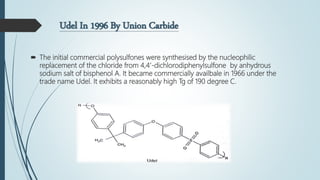
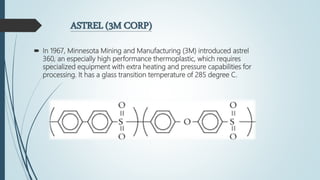
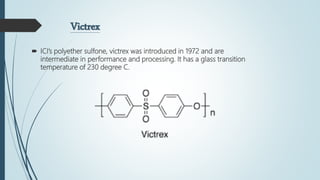
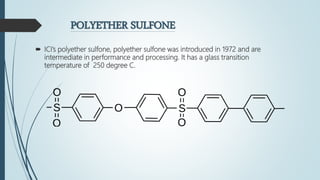
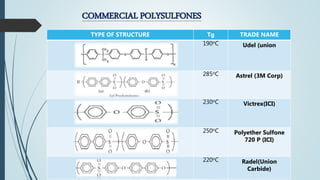
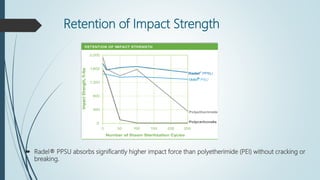
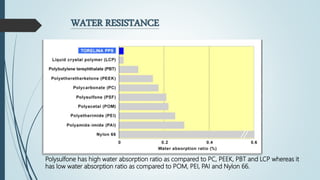
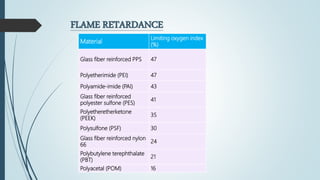
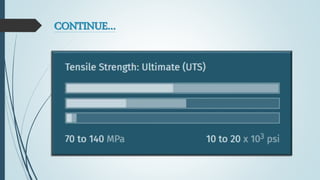
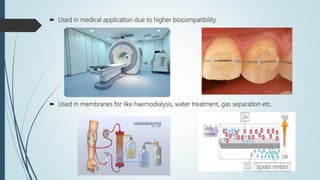
This document discusses the polymer polysulfone. It provides an introduction to polysulfone, describing its synthesis via polysulfonylation and polyetherification reactions. It discusses the production of major commercial polysulfones by Union Carbide, ICI, and 3M. The properties of polysulfone are summarized, including its high heat resistance, toughness, and chemical resistance. Applications are in electrical components, medical devices, automotive parts, and more due to these desirable properties. The advantages and few limitations of polysulfone are also outlined.