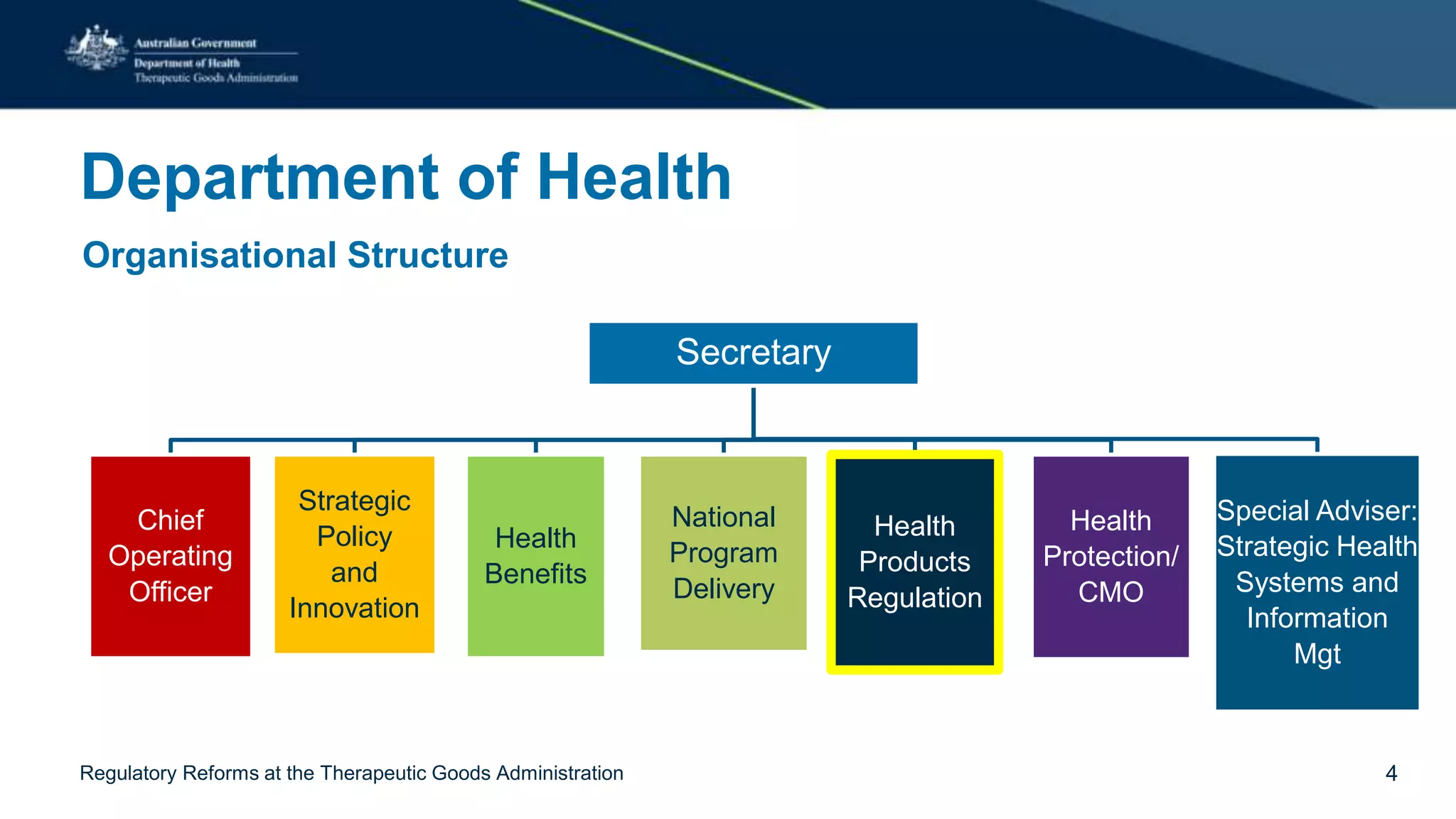
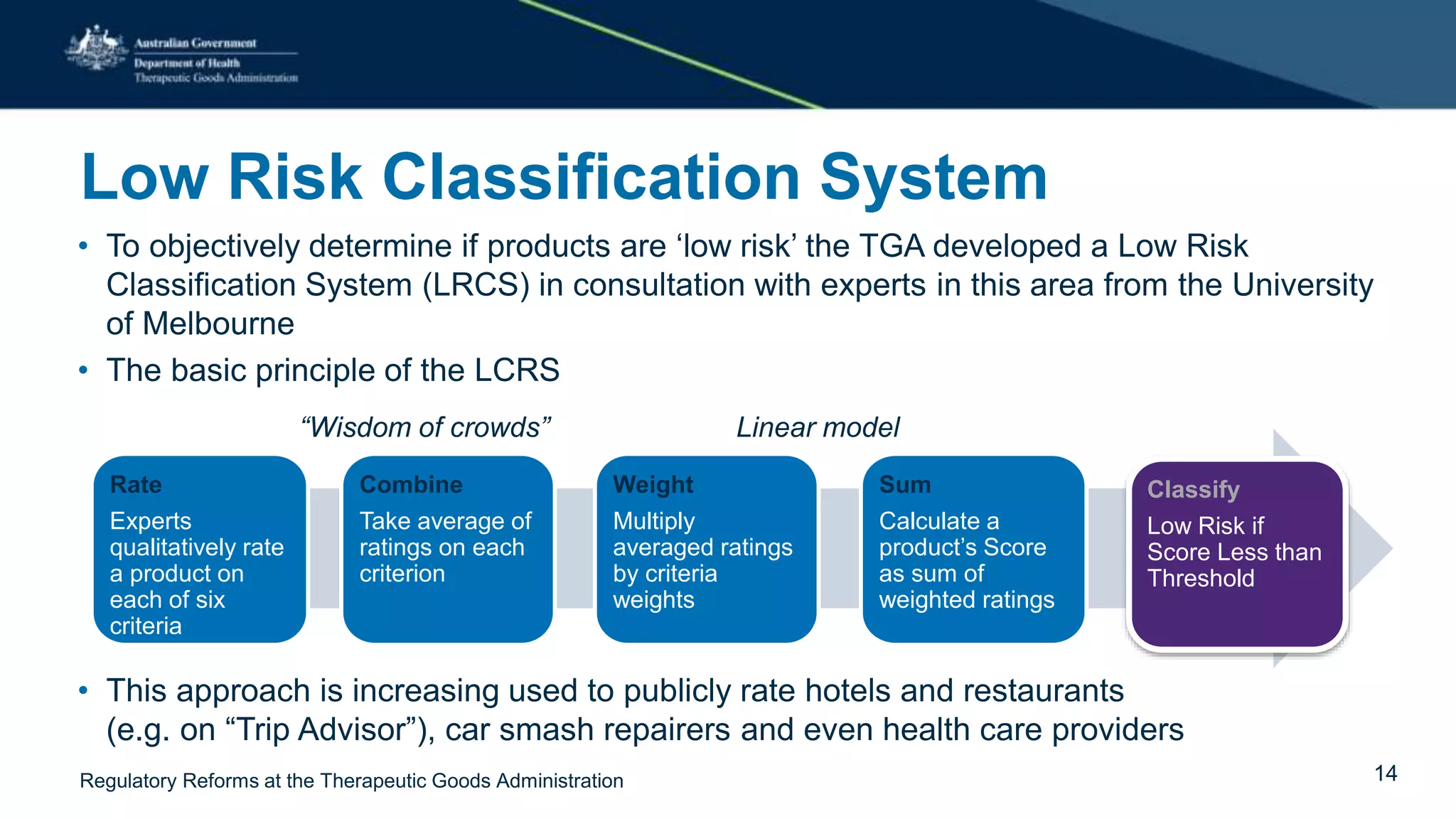
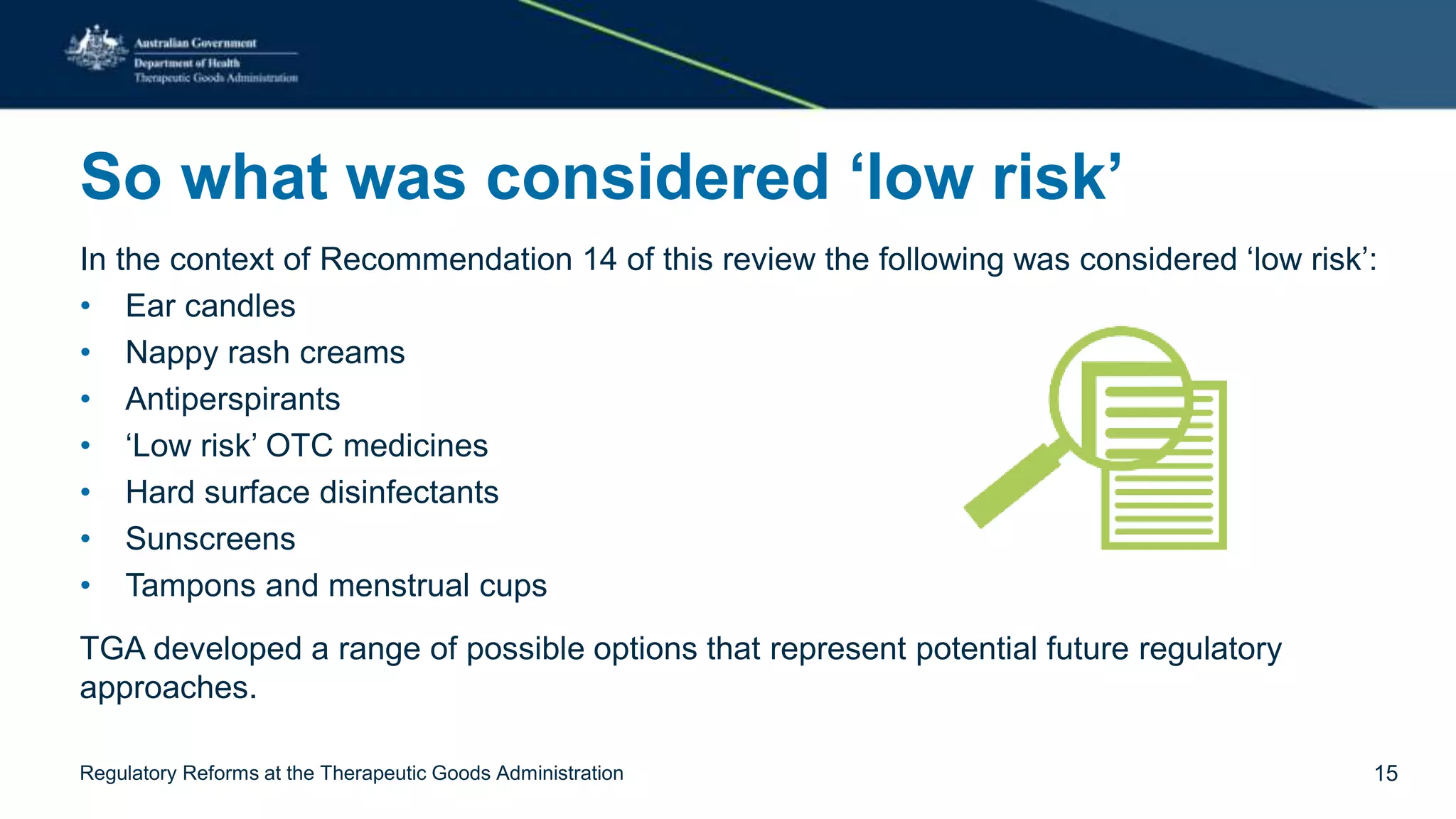
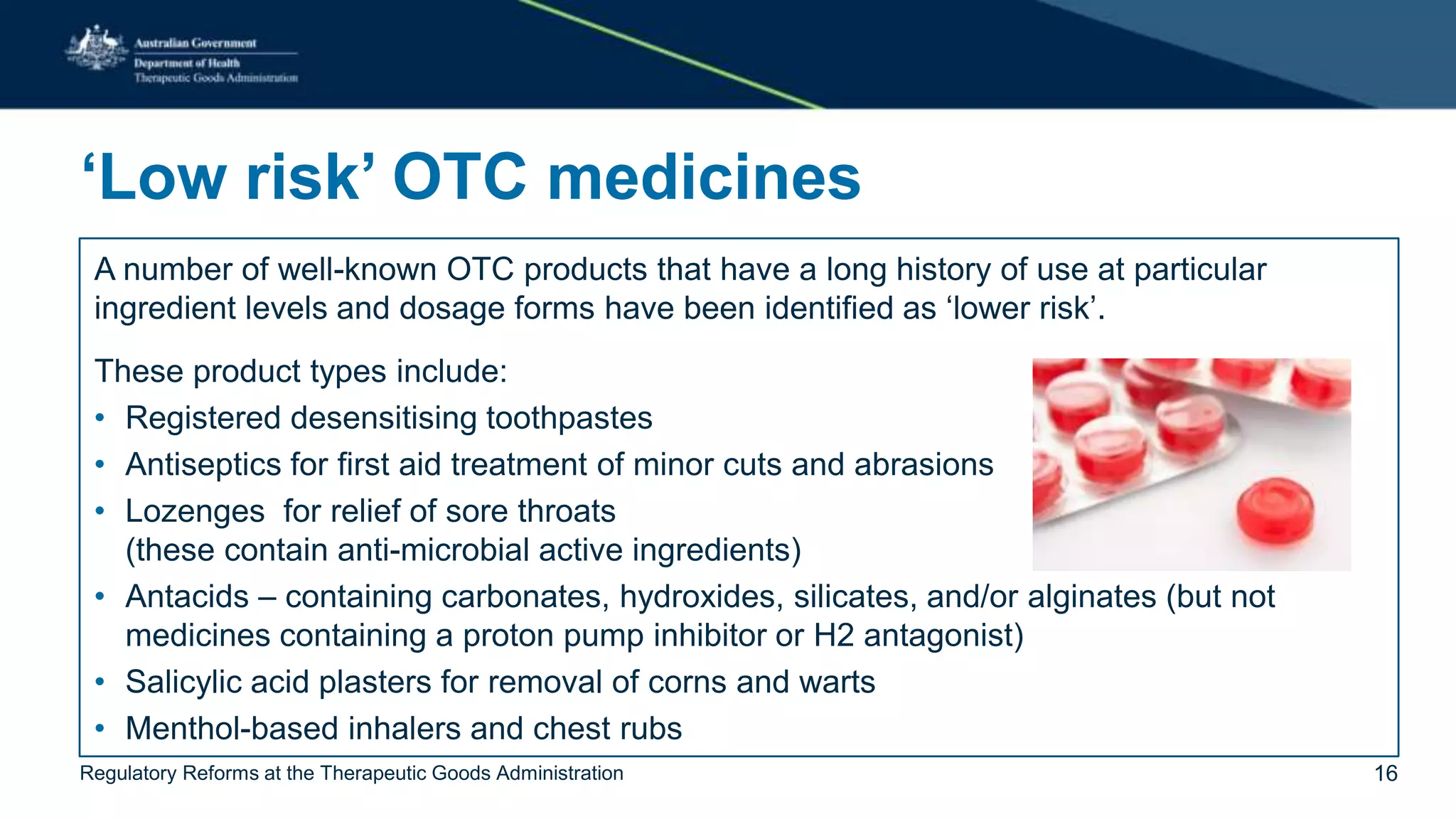
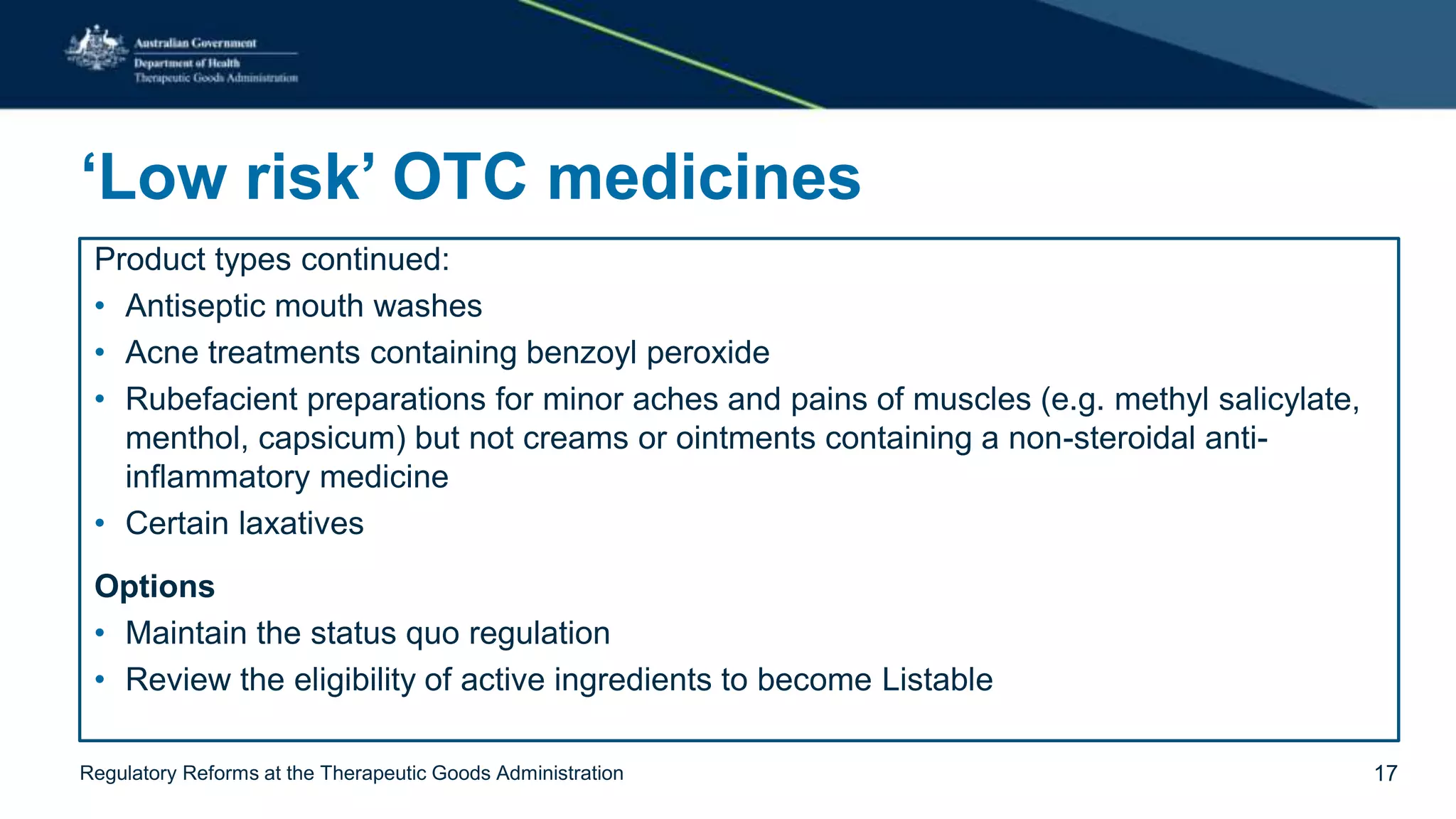
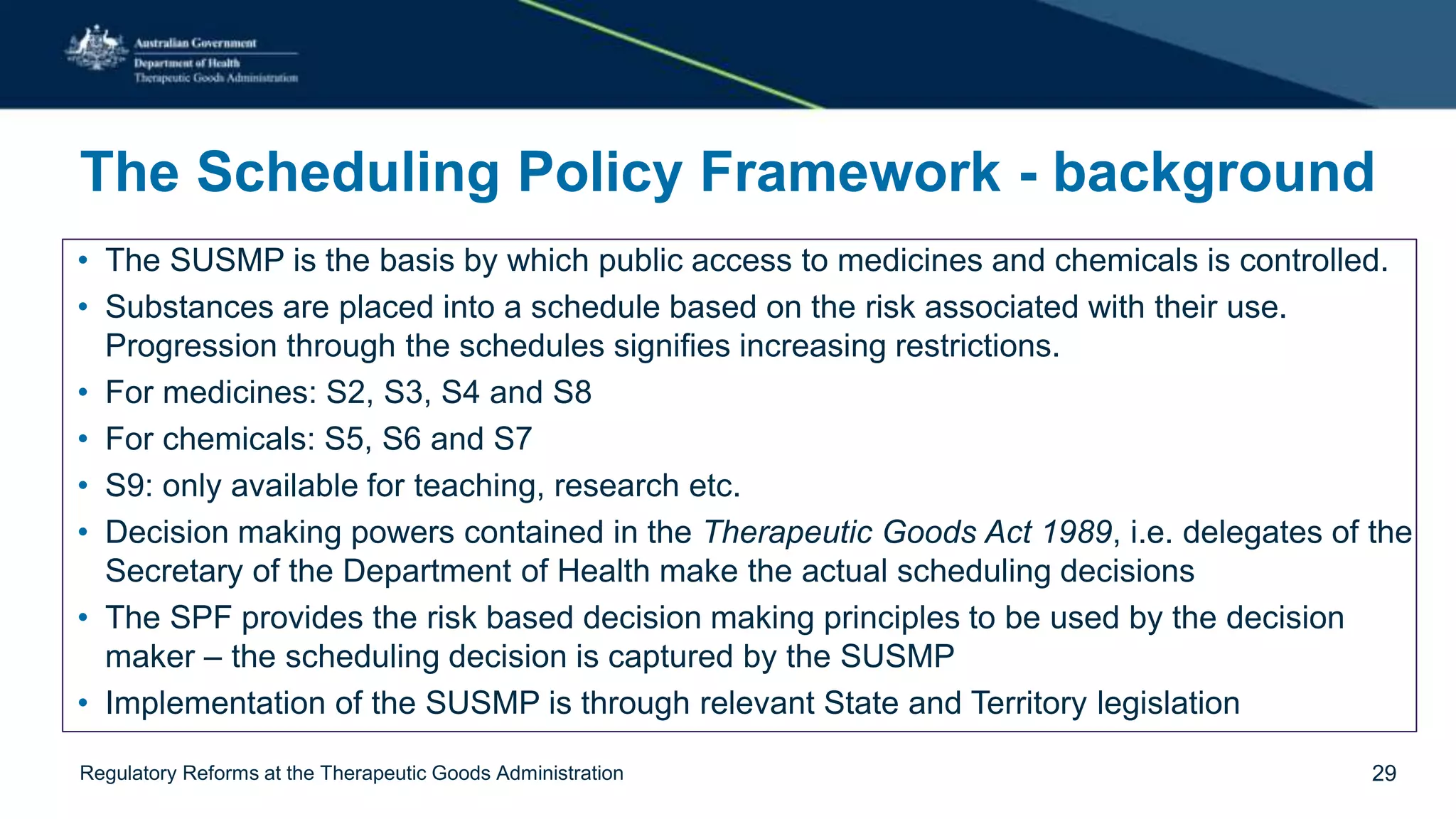
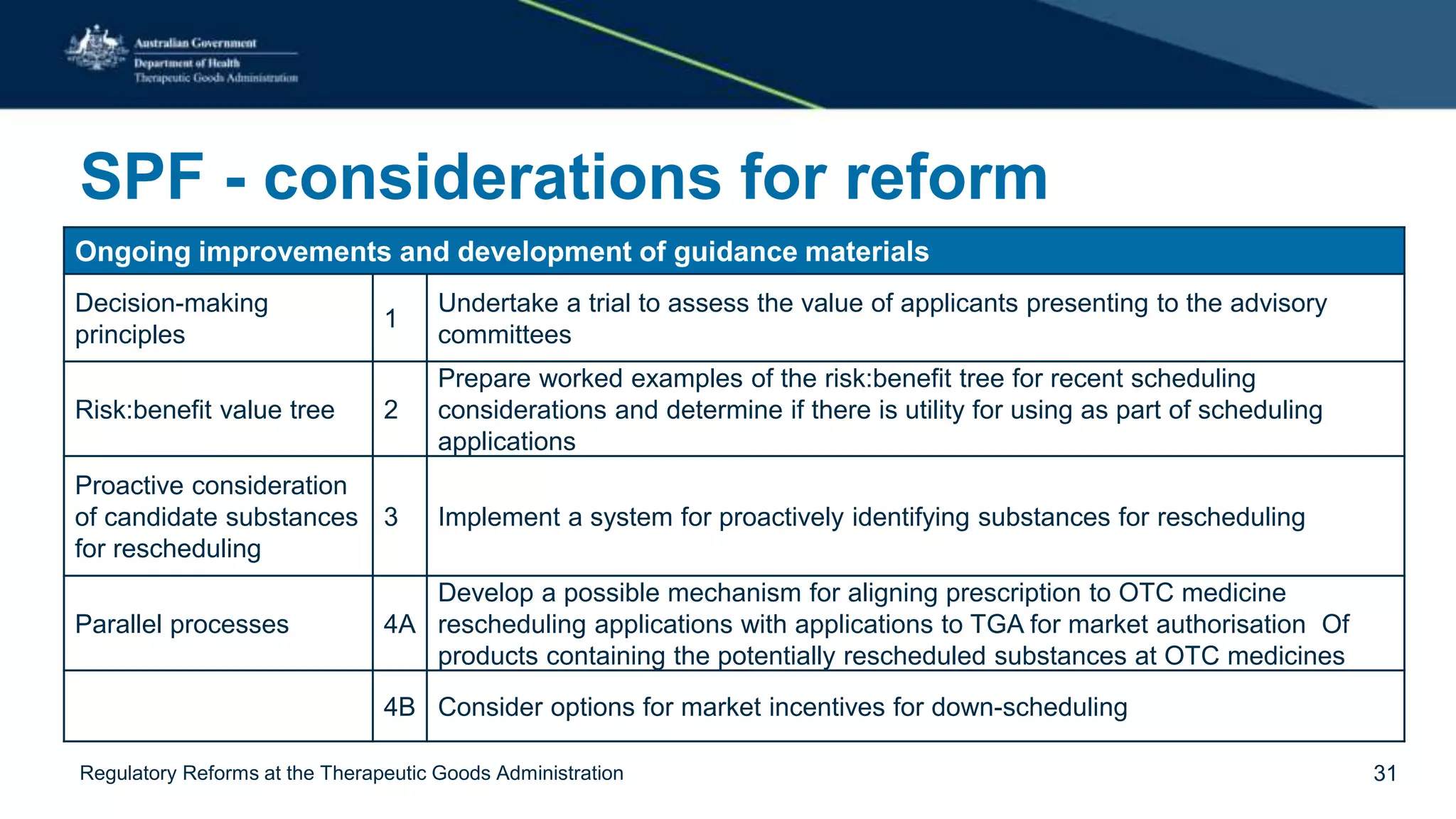
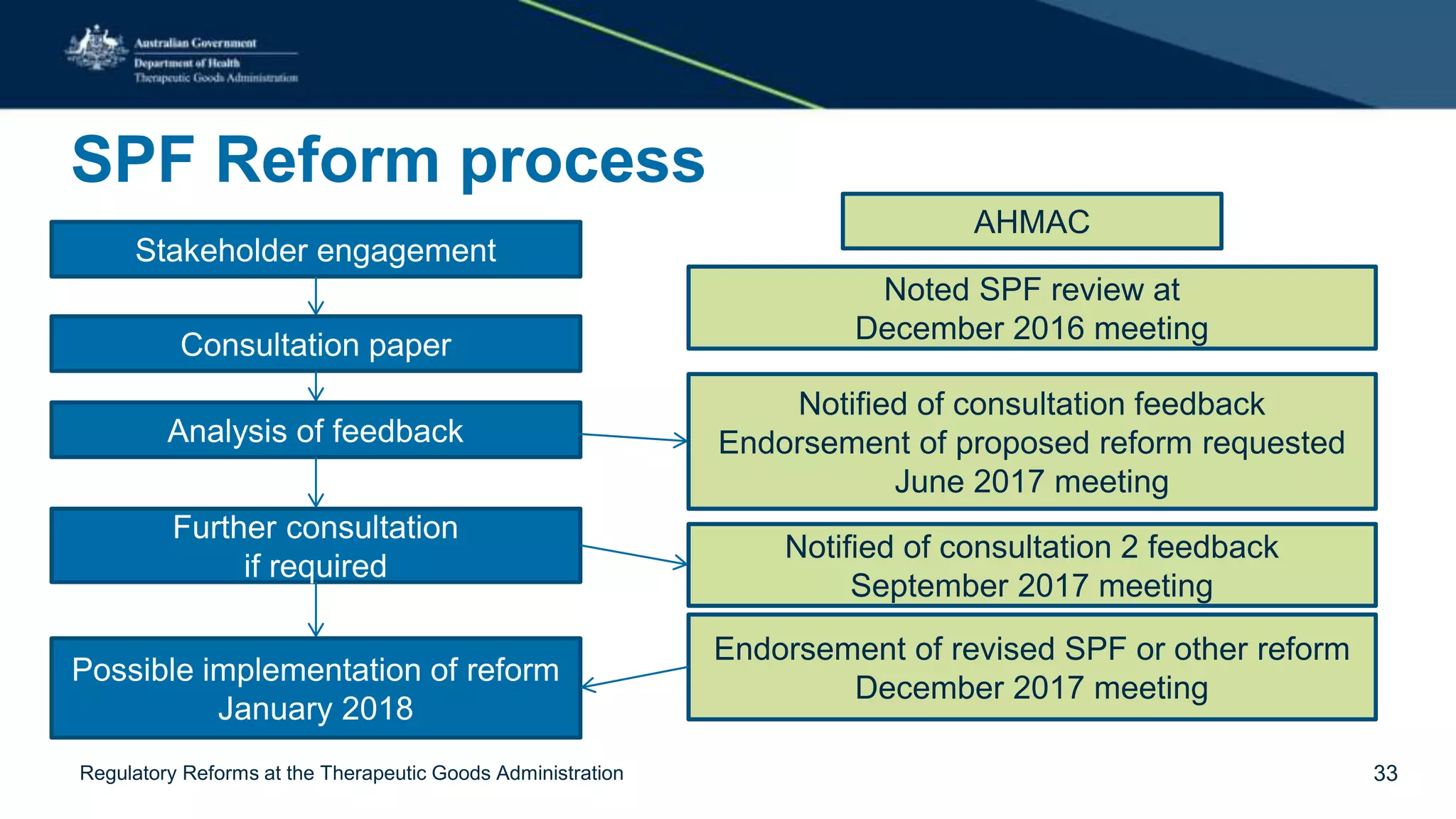
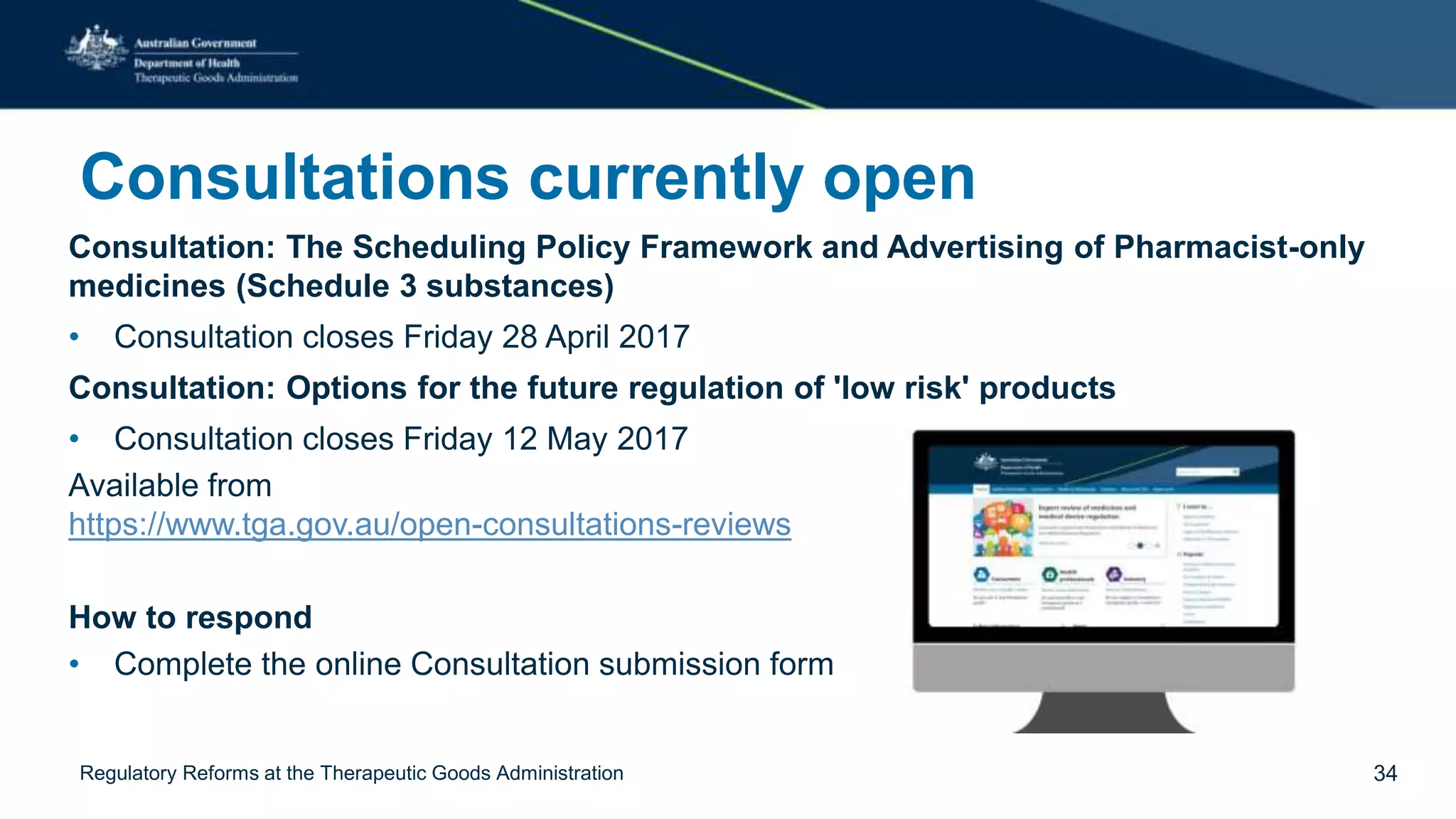
The document outlines regulatory reforms at the Therapeutic Goods Administration (TGA), covering its role in regulating therapeutic goods, risk management approaches, and ongoing consultations for low-risk product classifications. Key principles endorsed by the government include retaining responsibility for approval while introducing greater flexibility and alignment of regulations based on product risk. Future reforms focus on defining low-risk categories and improving regulatory frameworks for specific product types like sunscreens, vitamins, and medical devices.