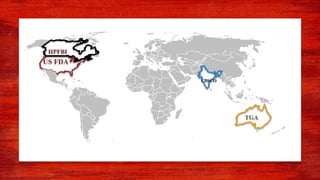
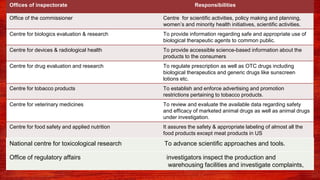
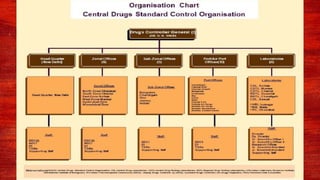
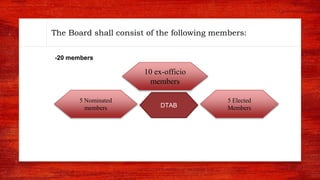
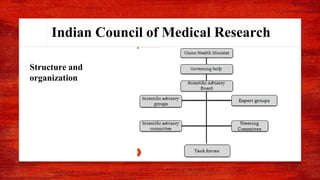
The document discusses various drug regulatory authorities around the world including the FDA, TGA, CDSCO, and ICMR. It provides an overview of the roles and responsibilities of these agencies in regulating drugs and pharmaceutical products to ensure public health and safety. Key functions of regulatory authorities that are described include evaluating new drugs, inspecting manufacturing facilities, monitoring adverse events, and establishing product standards.