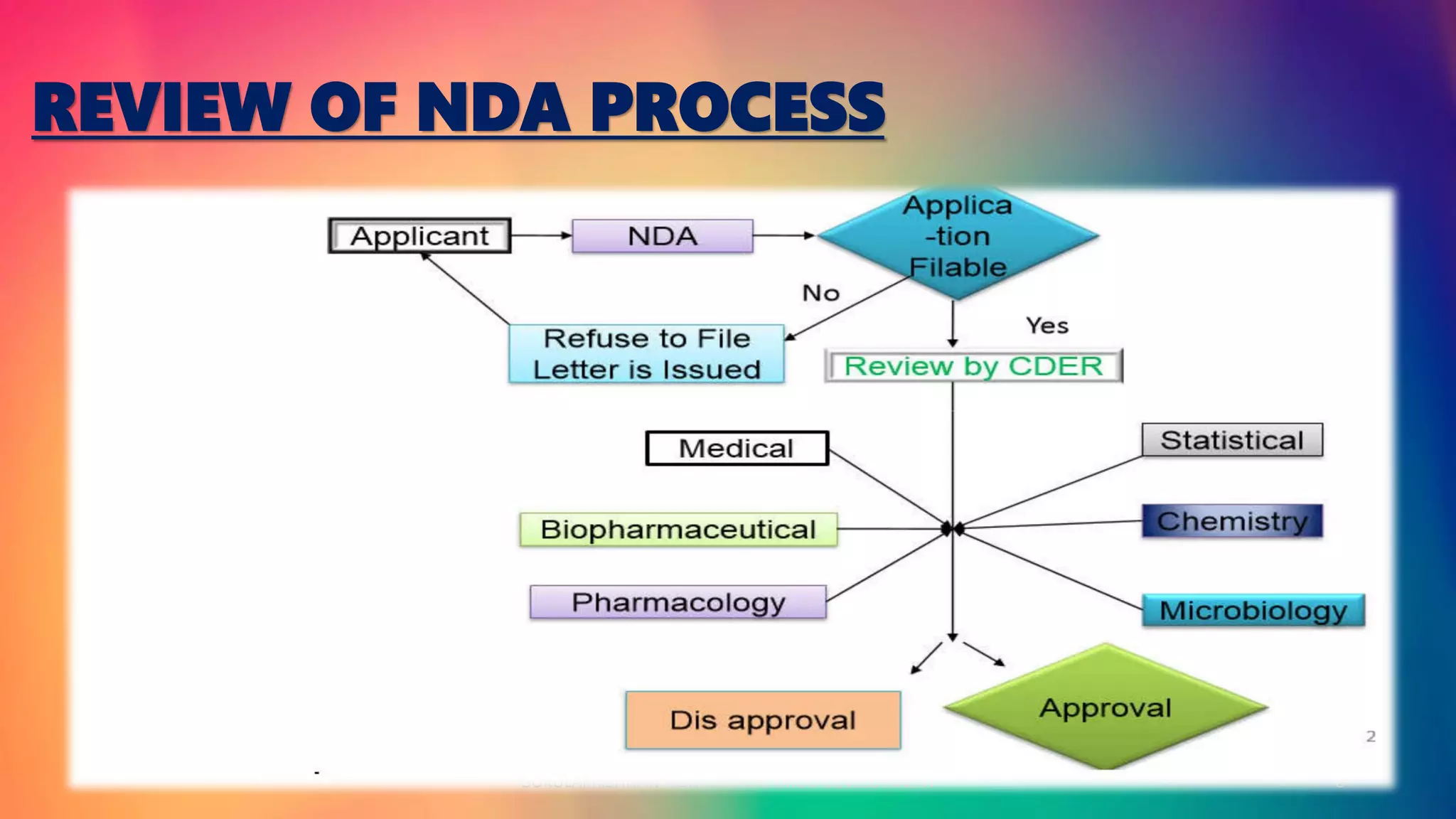
The document outlines the process and requirements for Non-Clinical Drug Development and New Drug Application (NDA) in the United States. It details the objectives of the NDA, its contents, regulatory guidelines, phases of clinical trials, and data required for FDA submission. The document serves as a comprehensive guide for drug sponsors to ensure compliance with FDA standards for safety, efficacy, and manufacturing quality.