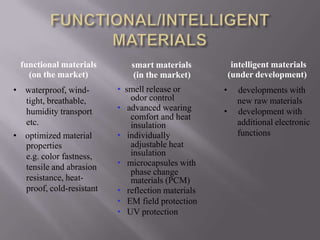
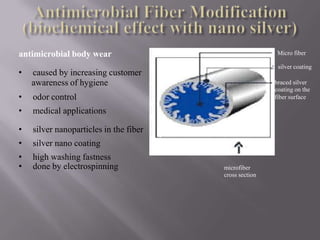
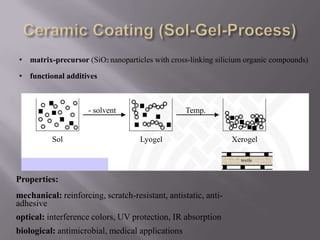
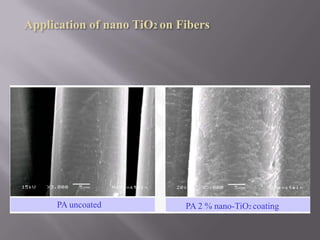
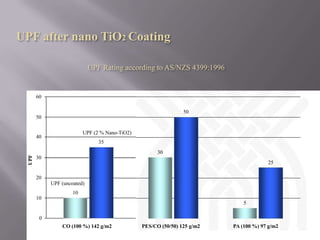
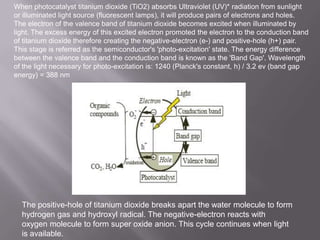
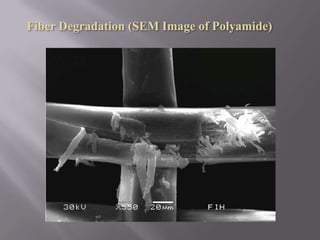
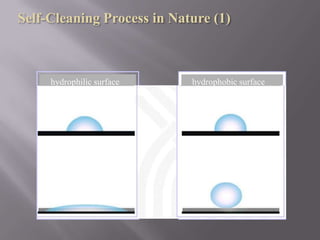
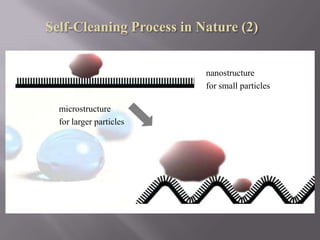
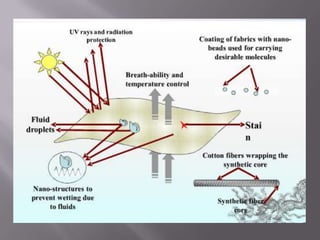
This document discusses applications of nanotechnology in textiles. It begins by defining nanotechnology as dealing with structures less than 100 nm. It describes how nano materials are used in pigments, computer chips, and surfaces to create properties like self-cleaning. Specific nanomaterials discussed for textiles include silver for antimicrobial effects, silicon dioxide for ceramic coatings, and titanium dioxide for UV protection and photocatalysis. The document also discusses functional materials for properties like waterproofing and breathability, as well as intelligent and smart materials under development. Examples of nanotechnology applications in textiles include antimicrobial silver coatings, ceramic coatings using sol-gel processes, and titanium dioxide coatings to improve ultraviolet protection. The document