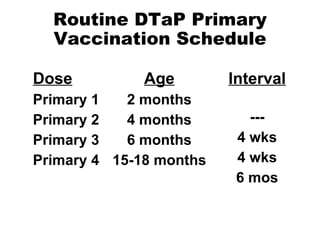
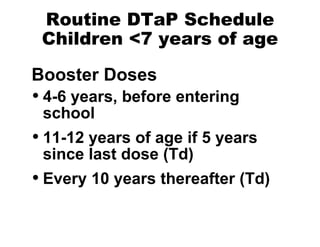
This document summarizes information about tetanus and tetanus toxoid vaccination. It describes tetanus as an illness caused by Clostridium tetani bacteria and its toxins. It also outlines tetanus clinical features, epidemiology, prevention through proper wound management and vaccination, and vaccination schedules and booster recommendations for routine DTaP, Td, and tetanus toxoid administration in both children and adults.









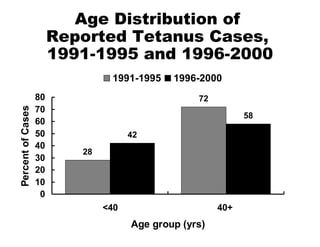
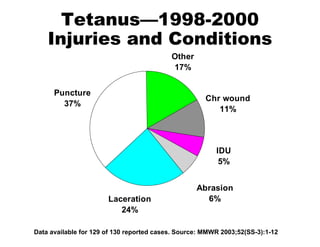
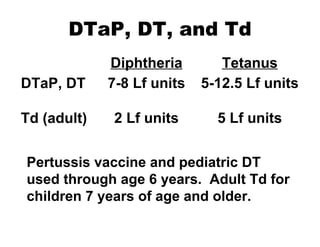
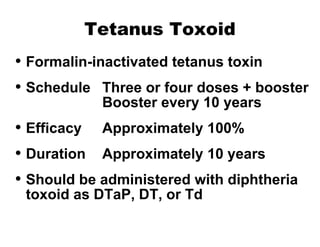




![National Immunization Program Hotline 800.232.2522 Email [email_address] Website www.cdc.gov/nip](https://image.slidesharecdn.com/tetanus8p-091002100151-phpapp02/85/Tetanus8p-24-320.jpg)