- Influenza is a highly infectious viral illness that causes annual epidemics and occasional pandemics, with the 1918 pandemic resulting in an estimated 21 million deaths worldwide.
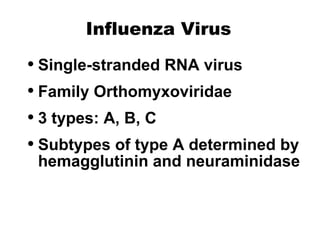
- Influenza viruses are classified into types A, B, and C, with types A and B responsible for seasonal epidemics. Type A viruses can undergo antigenic shifts or drifts, resulting in pandemics or epidemics respectively.
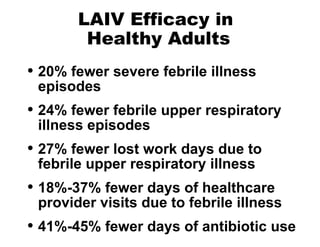
- Influenza vaccination is recommended for many high-risk groups to reduce hospitalizations and deaths from influenza. Both inactivated and live attenuated vaccines are available, with inactivated vaccines recommended for young children, elderly, and immunocompromised individuals.


















































![National Immunization Program Hotline 800.232.2522 Email [email_address] Website www.cdc.gov/nip](https://image.slidesharecdn.com/influenza8p-091002100201-phpapp02/85/Influenza8p-53-320.jpg)