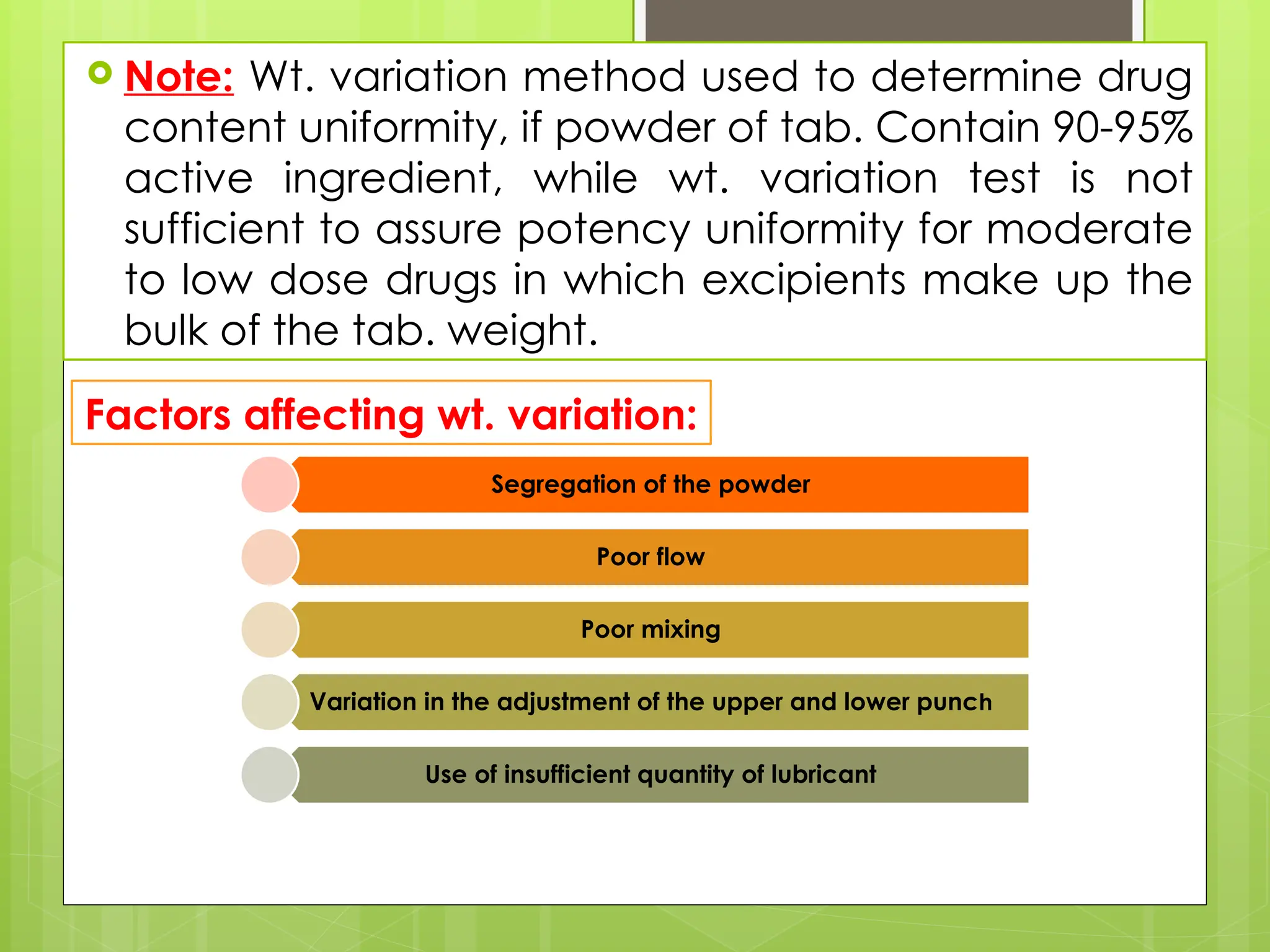
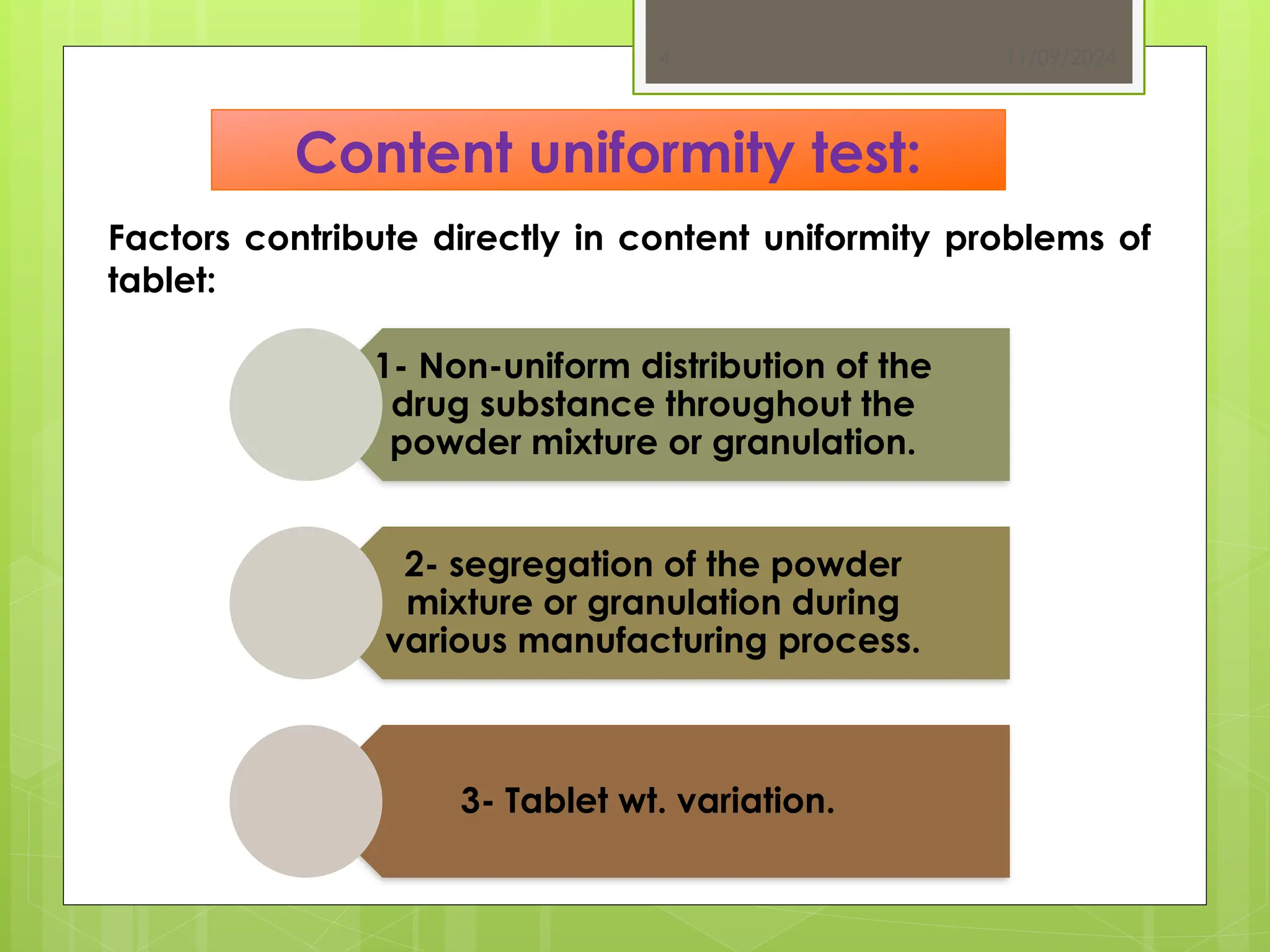
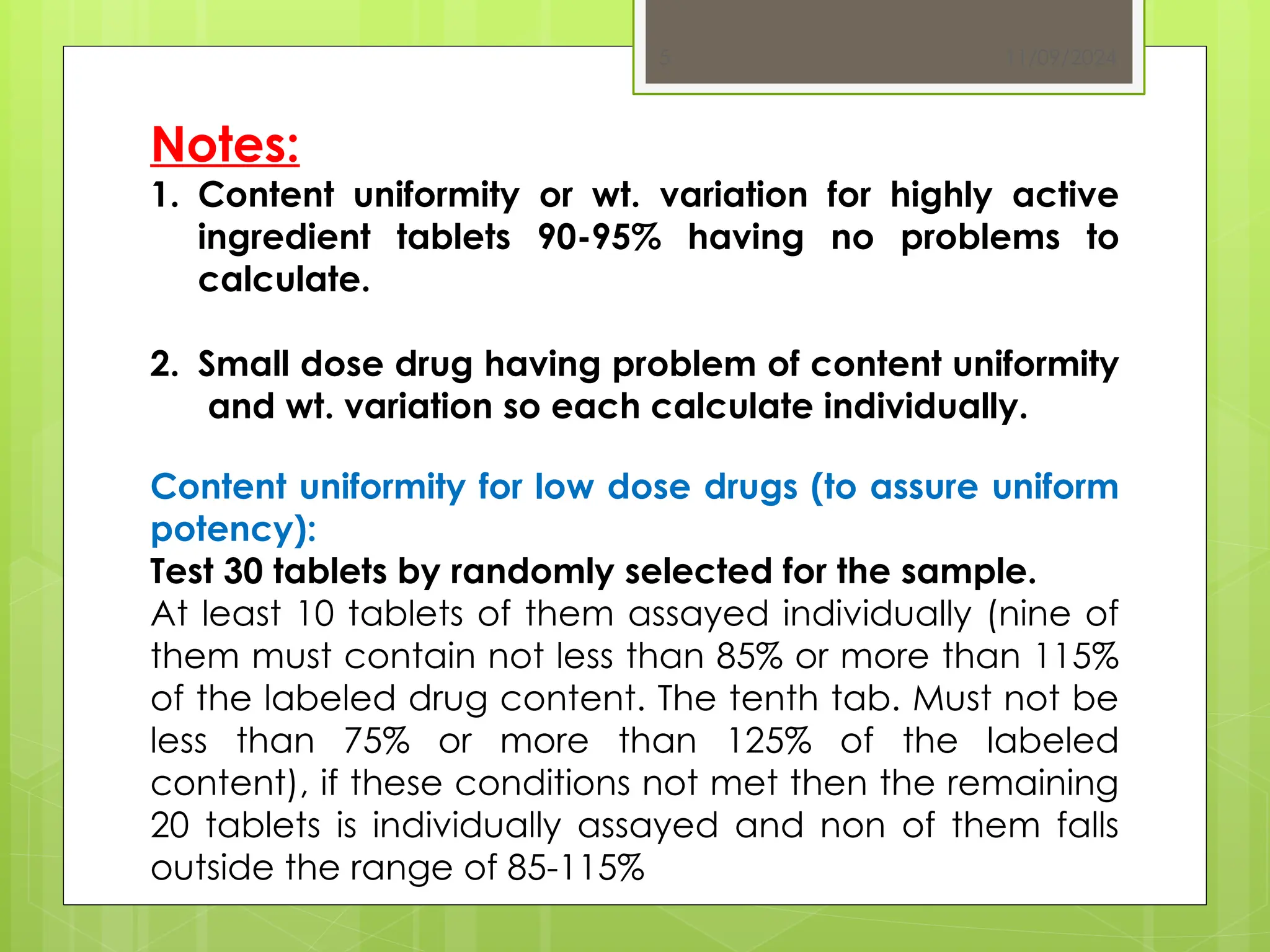
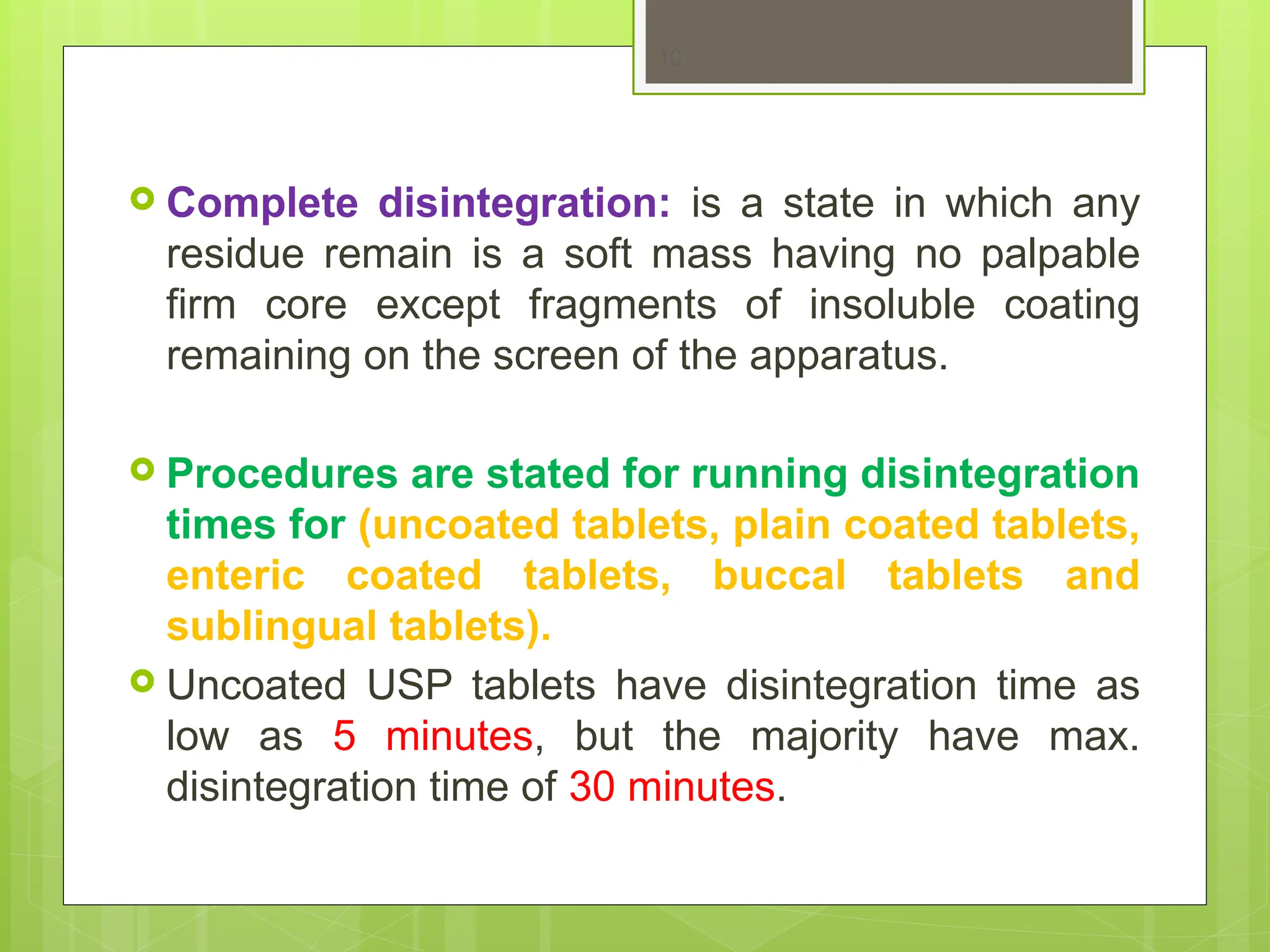
The document outlines the official testing procedures for weight variation, content uniformity, and disintegration of tablets according to USP guidelines. It specifies methods for assessing weight variations in tablets based on their average weight, factors affecting uniformity, and conditions that must be met for content uniformity tests. Additionally, it details disintegration testing processes for various types of tablets to ensure they dissolve effectively, emphasizing the relationship between disintegration time and drug availability.