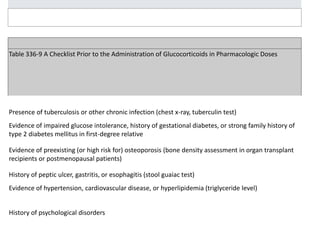
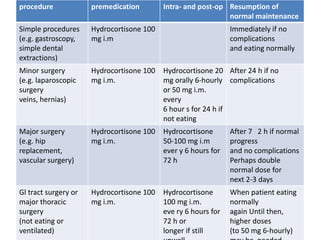
The document discusses systemic steroids, including:
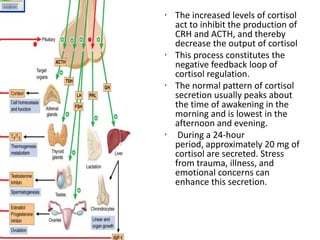
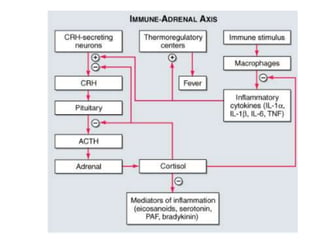
1. Steroids are produced by the adrenal cortex and include glucocorticoids, mineralocorticoids, and androgens which are derived from cholesterol.
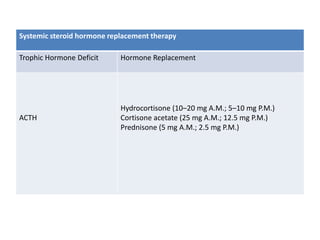
2. Common therapeutic uses of glucocorticoids include respiratory diseases like asthma, rheumatological diseases, and as anti-inflammatory drugs.
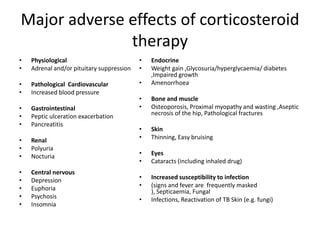
3. Long term steroid use can cause adverse effects like weight gain, high blood pressure, easy bruising, infections, osteoporosis, and psychiatric issues like depression. Regular monitoring is important with steroid therapy.