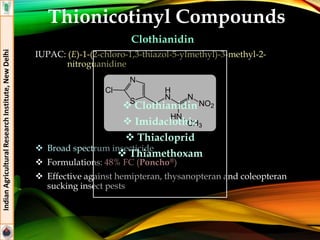
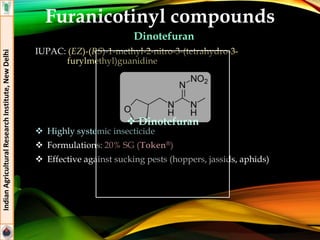
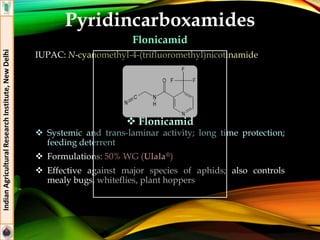
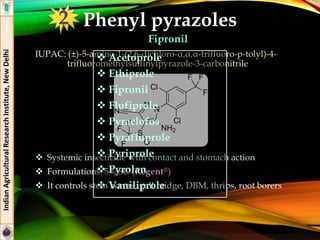
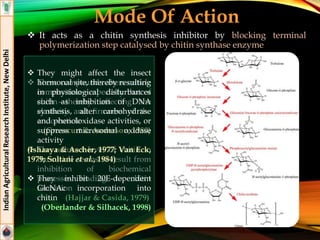
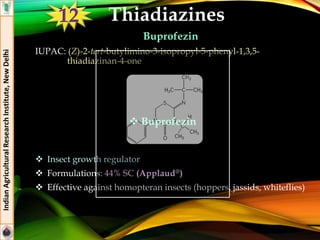
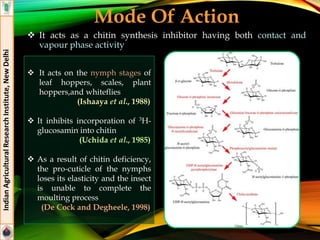
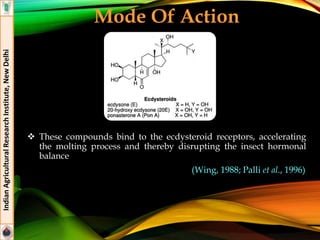
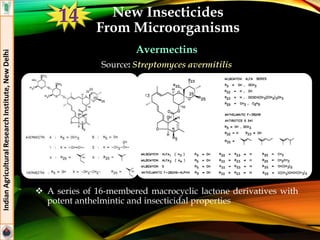
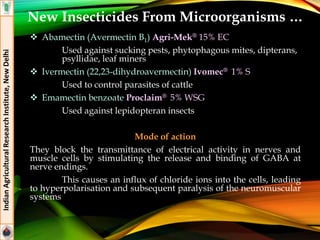
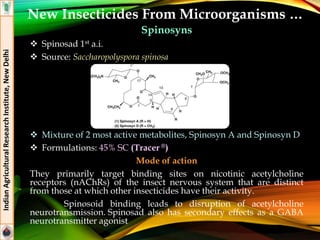
The document provides an overview of various insecticides used in agriculture, discussing their classifications, effectiveness, and modes of action. It details older insecticide classes and highlights new chemistry, including neonicotinoids and other innovative compounds derived from microorganisms. Additionally, it describes their targeted applications against pests affecting crops, emphasizing their selective toxicity and chemical properties.








![Oxadiazines
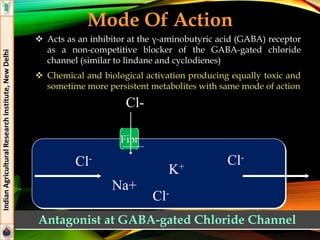
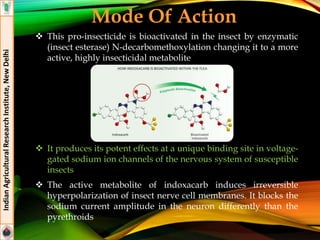
Indoxacarb
IUPAC: Methyl 7-chloro-2,5-dihydro-2-[[(methoxycarbonyl)[4-
(trifluoromethoxy)phenyl]amino]carbonyl]indeno[1,2-e]
[1,3,4]oxadiazine-4a(3H)-carboxylate
Formulations: 15.8% EC (Avaunt®)
Effective against lepidopteran pests (American boll worm,
DBM, Helicoverpa armigera, Plutella xylostella)
IndianAgriculturalResearchInstitute,NewDelhi
Indoxacarb
3](https://image.slidesharecdn.com/prithusemchemphd-140719063056-phpapp01/85/New-Chemistries-For-Insect-Management-17-320.jpg)
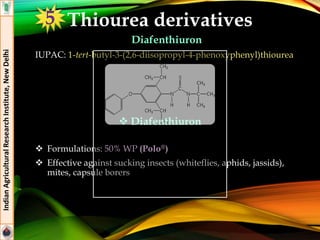




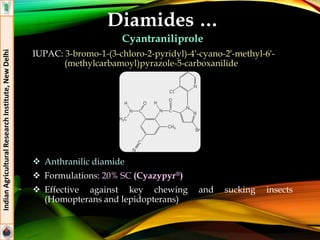
![Diamides
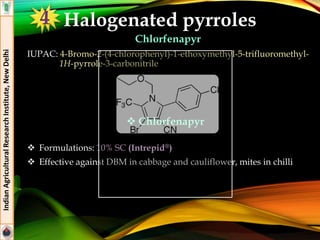
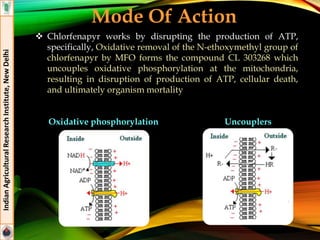
Flubendiamide
IUPAC: 3-iodo-N’-(2-mesyl-1,1-dimethylethyl)-N-{4-[1,2,2,2-
tetrafluoro-1-trifluoromethyl)ethyl] -o-tolyl} phthalamide
Pthalic diamide
Formulations: 20% WG (Takumi®), 39.35% SC (Fame®)
Effective against insect pests of rice (stem borer. Leaf folder) and
cotton (Helicoverpa armigera, spotted boll worm)
IndianAgriculturalResearchInstitute,NewDelhi
Flubendiamide
Chlorantraniliprole
Cyantraniliprole
Cyclaniliprole
Tetraniliprole
10](https://image.slidesharecdn.com/prithusemchemphd-140719063056-phpapp01/85/New-Chemistries-For-Insect-Management-27-320.jpg)

![Flufenoxuron
IUPAC: 1-[4-(2-chloro-α,α,α-trifluoro-p-tolyloxy)-2-fluorophenyl]-
3-(2,6-difluorobenzoyl)urea
Insect growth regulator
Formulations: 10% DC (Cascade®)
Effective against immature stages of many phytophagous mites
and insects pests of pome fruit, vines, citrus, tea, ornamentals
IndianAgriculturalResearchInstitute,NewDelhi
Benzoyl Urea
Bistrifluron
Chlorbenzuron
Chlorfluazuron
Dichlorbenzuron
Diflubenzuron
Flucycloxuron
Flufenoxuron
Hexaflumuron
Lufenuron
Novaluron
Noviflumuron
Penfluron
Teflubenzuron
11](https://image.slidesharecdn.com/prithusemchemphd-140719063056-phpapp01/85/New-Chemistries-For-Insect-Management-31-320.jpg)





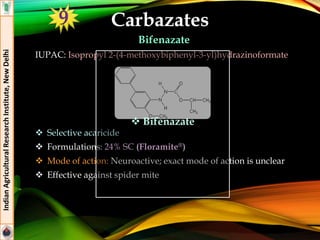
![Tetronic Acid Derivatives
Spiromesifen
IUPAC: 3-mesityl-2-oxo-1-oxaspiro[4.4]non-3-en-4-yl 3,3-
dimethylbutyrate
Formulations: 24% SC (Oberon®)
Mode of action: Prevent Lipid biosynthesis by inhibiting acetyl
CoA carboxylase
Effective against mites and moth scale insects
IndianAgriculturalResearchInstitute,NewDelhi
Spiromesifen
16](https://image.slidesharecdn.com/prithusemchemphd-140719063056-phpapp01/85/New-Chemistries-For-Insect-Management-44-320.jpg)
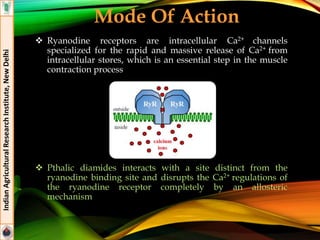
![Sulfoximines
Sulfoxaflor
IUPAC: [methyl(oxo){1-[6-(trifluoromethyl)-3-pyridyl]ethyl}-
λ6-sulfanylidene]cyanamide
Systemic insect neuro-toxin
Formulations: 24% SC (Transform®)
Effective against major species of sap feeding insects
IndianAgriculturalResearchInstitute,NewDelhi
Sulfoxaflor
17](https://image.slidesharecdn.com/prithusemchemphd-140719063056-phpapp01/85/New-Chemistries-For-Insect-Management-45-320.jpg)