This document summarizes a symposium on paediatric cataracts presented by Dr. Ganesh Pillay. Some key points:
- Paediatric cataracts are a major cause of preventable childhood blindness worldwide.
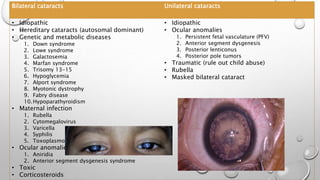
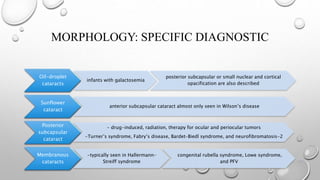
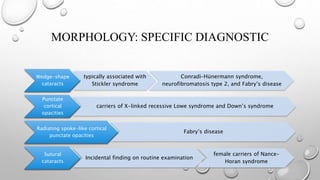
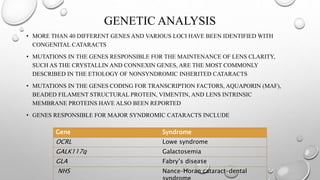
- Cataracts in children can be bilateral or unilateral, and have many potential causes including genetic conditions, infections, trauma and metabolic disorders.
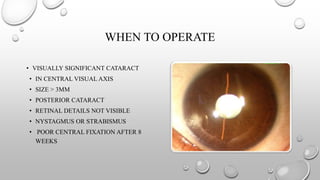
- A thorough history and examination is important to determine the type and severity of the cataract. Investigations may be needed depending on clinical findings.
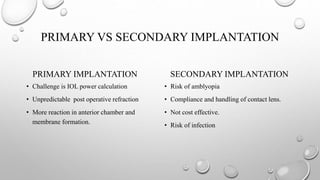
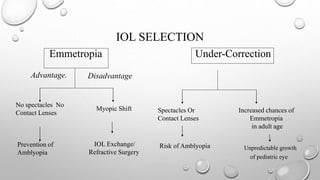
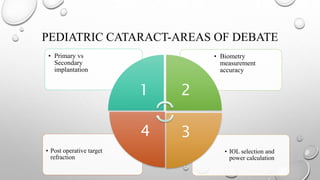
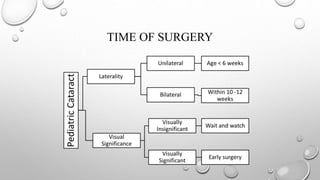
- The timing and type of cataract surgery in children presents various challenges and debates regarding issues like IOL selection and post-operative management.
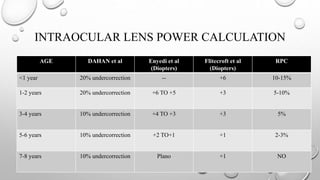
- Studies like the Inf


















![RESULT
• IOL POWER AND PLACEMENT
• THE MEAN POWER (±SD) OF THE IMPLANTED IOL WAS 29.9 (5.7) D OVERALL (31.5
[5.0] D FOR THE YOUNGER AGE GROUP AND 28.7 [6.0] D FOR THE OLDER AGE
GROUP); IOL POWER RANGE WAS 11.5 TO 40.0 D.
• FOLLOW-UP REFRACTION AND PREDICTION ERROR (PE) THE OVERALL MEAN
(±SD) REFRACTION AT 1 MONTH WAS +6.1 (2.0) D. THE MEAN REFRACTION WAS
+6.6 (1.9) D IN THE YOUNGER AGE GROUP AND +5.7 (1.9) D IN THE OLDER AGE
GROUP.](https://image.slidesharecdn.com/symposiumpediatriccataract-170720190142/85/Symposium-pediatric-cataract-29-320.jpg)