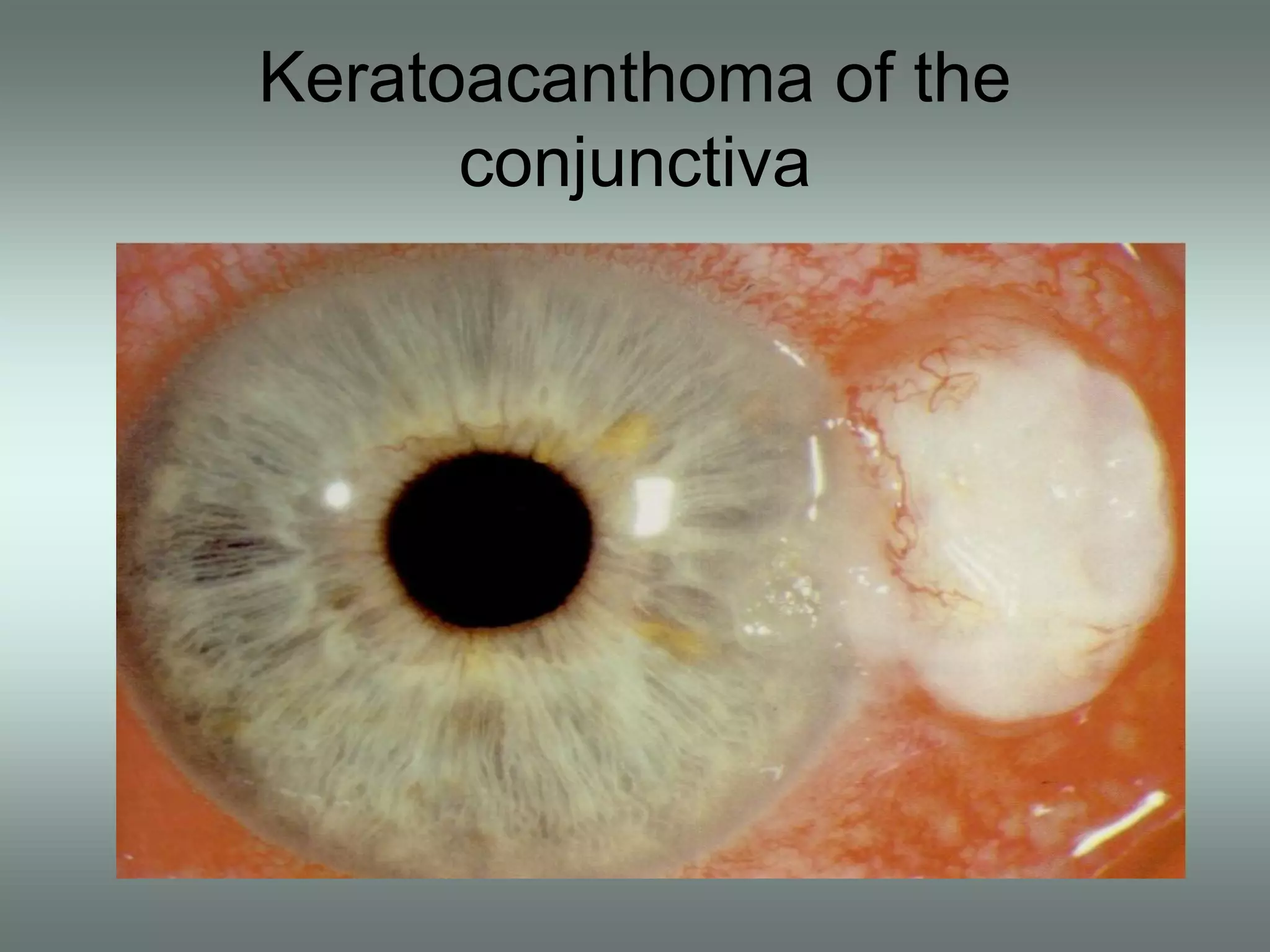
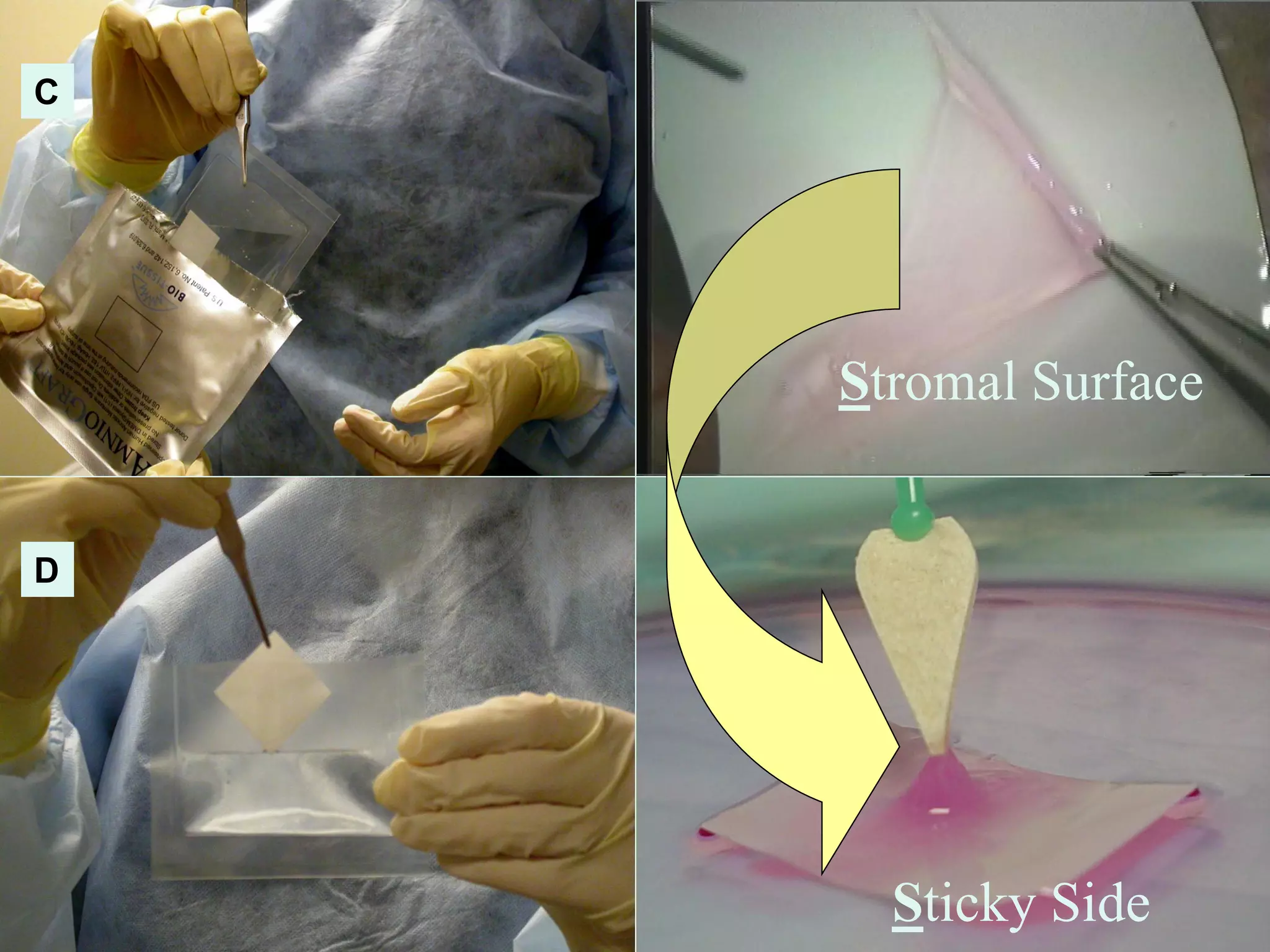
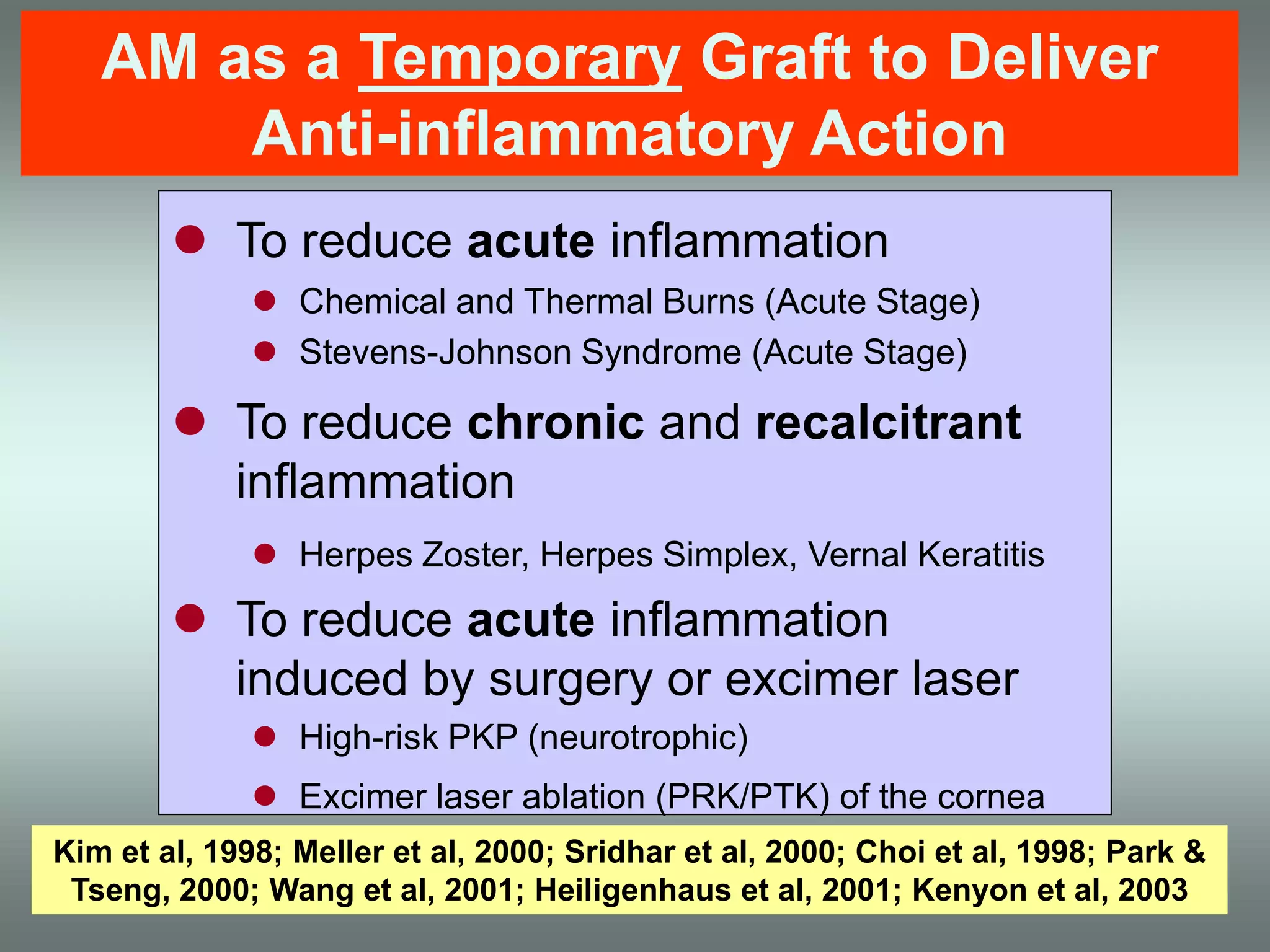
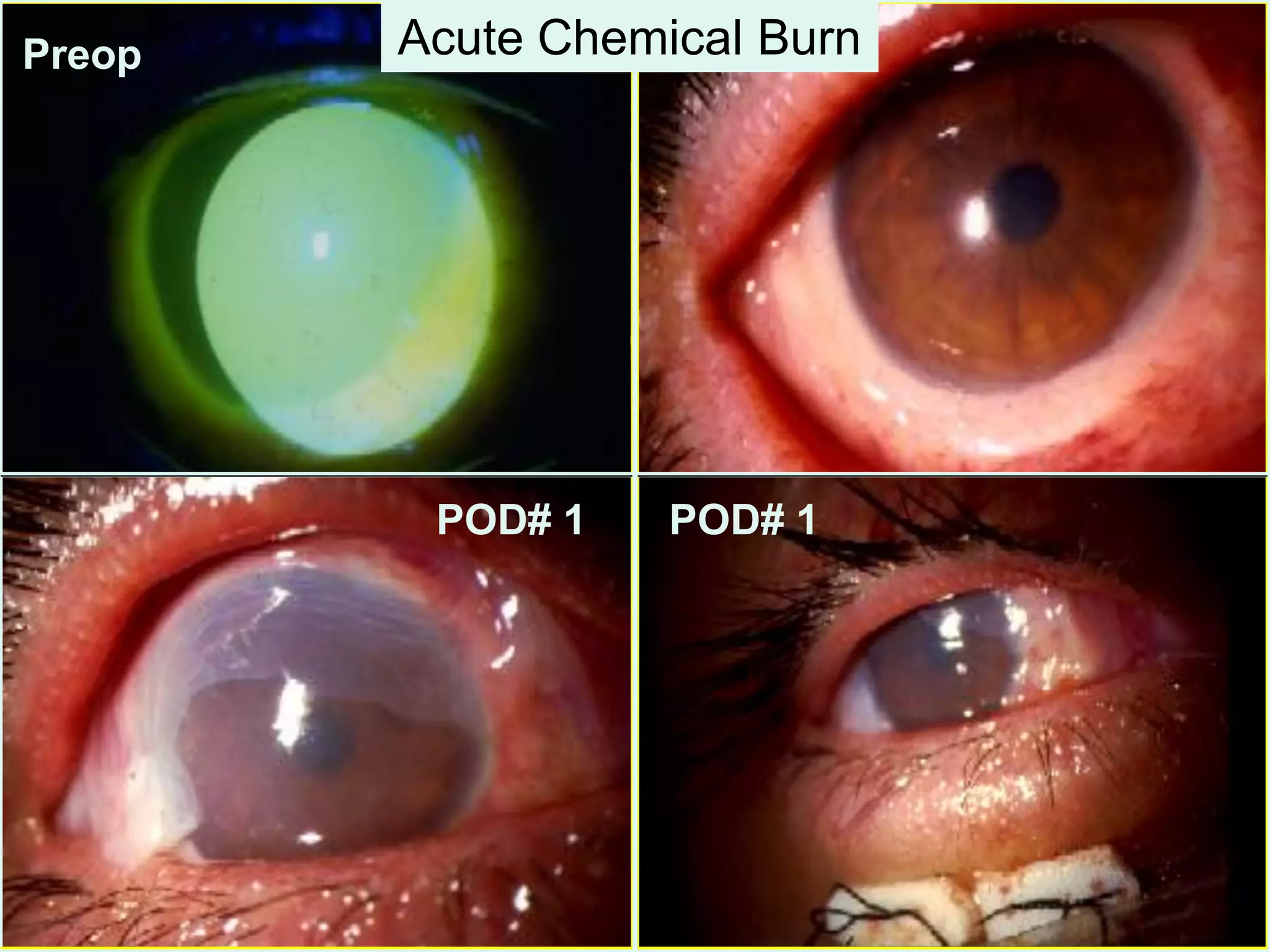
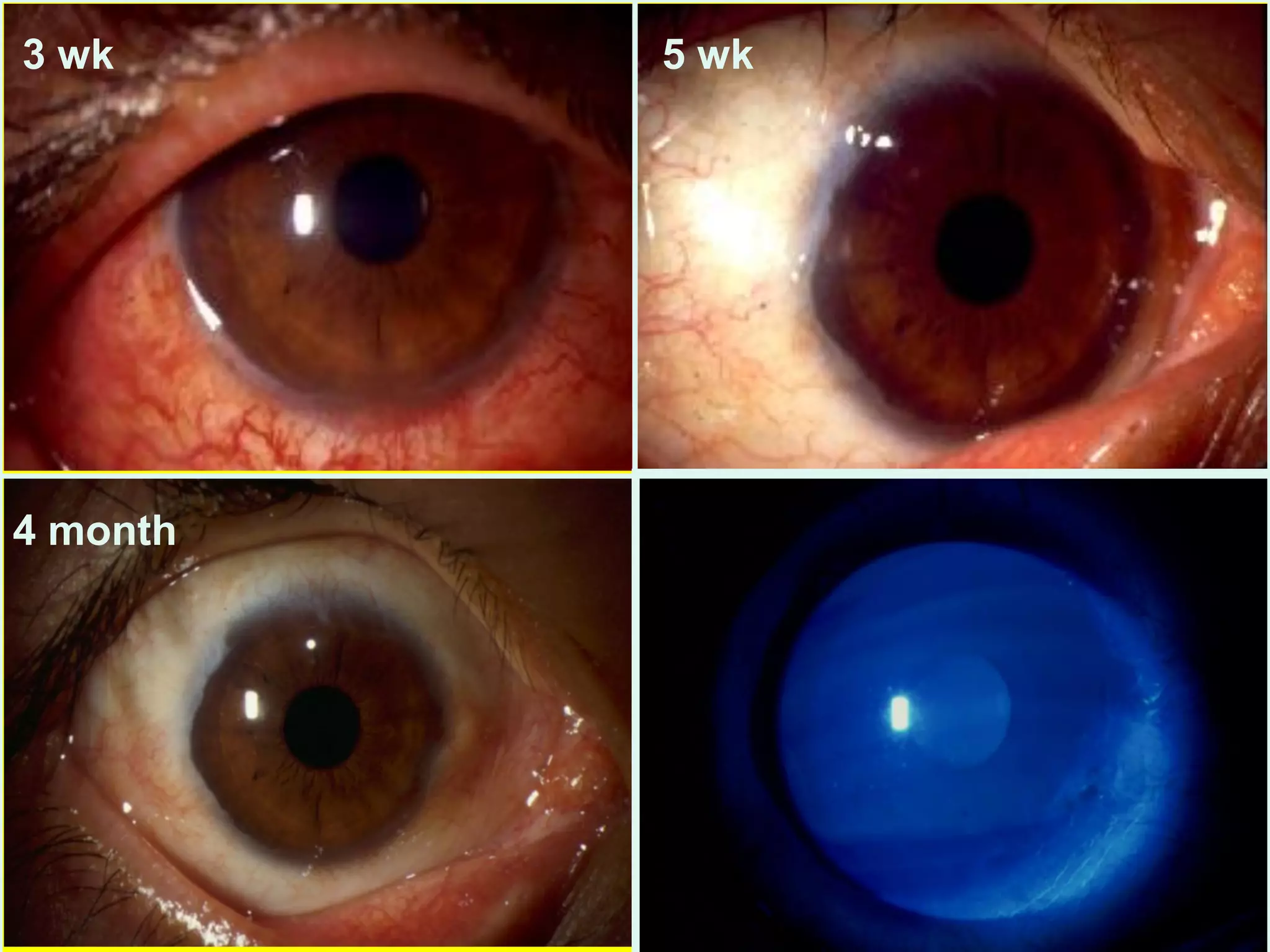
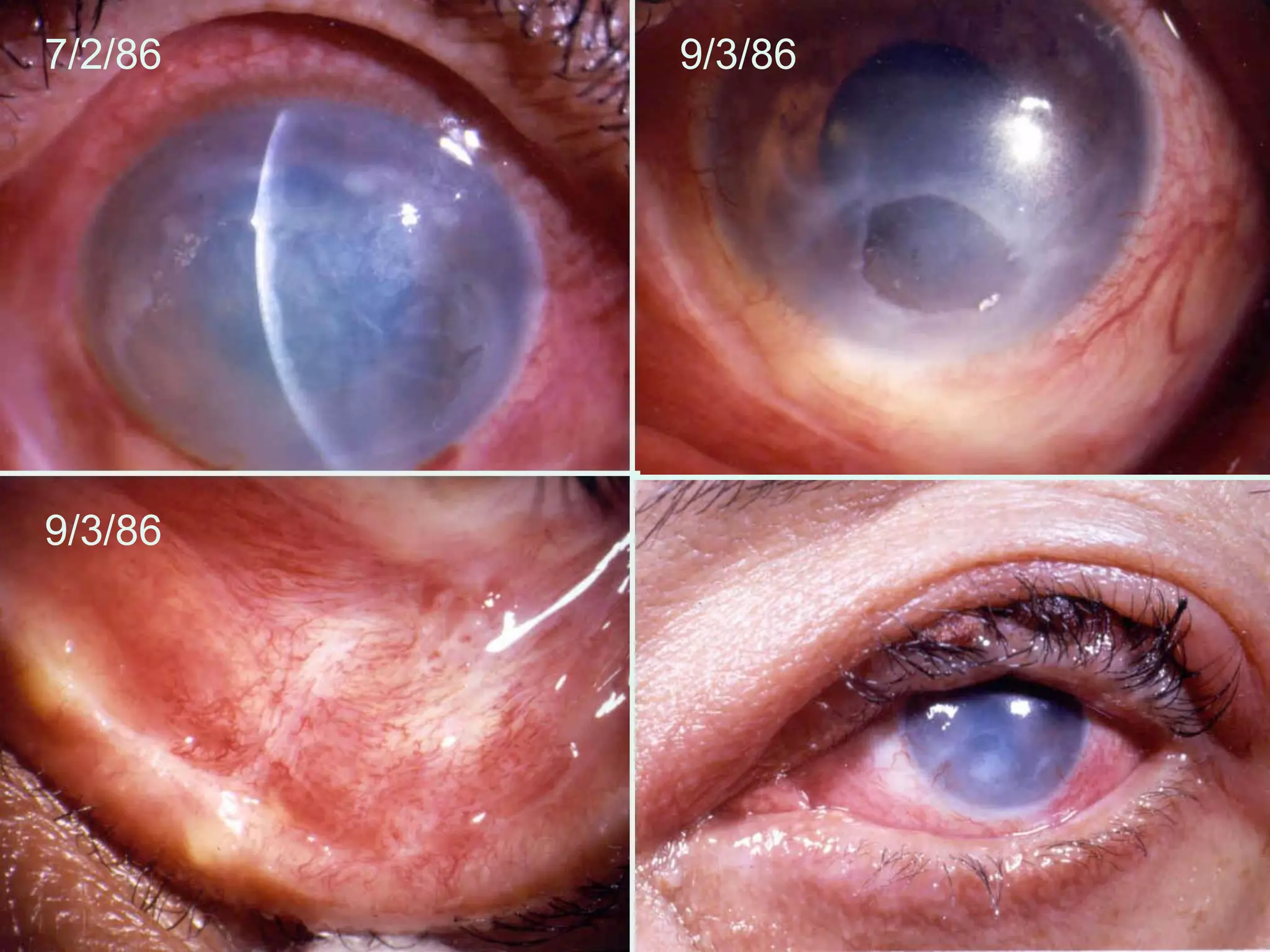
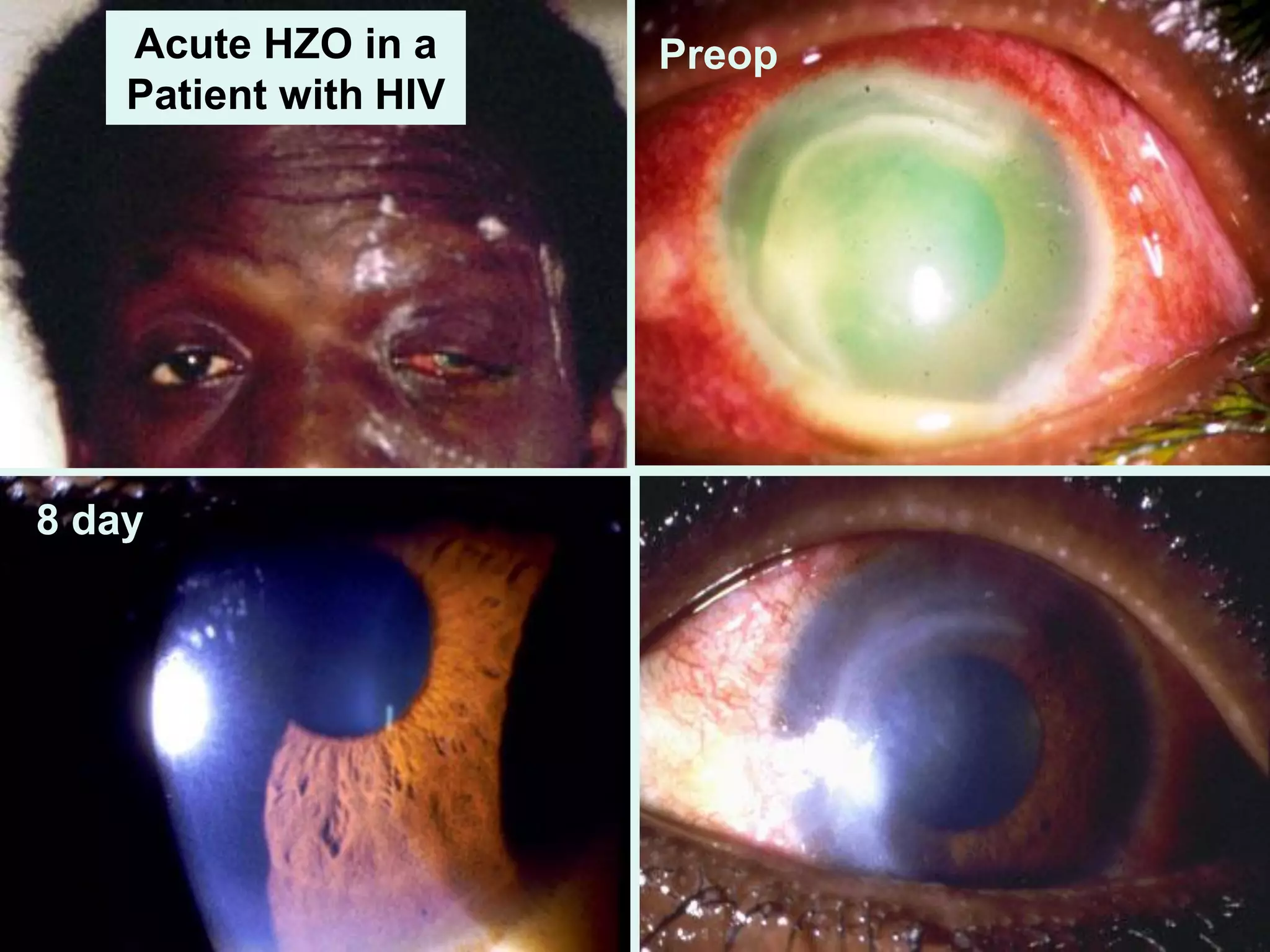
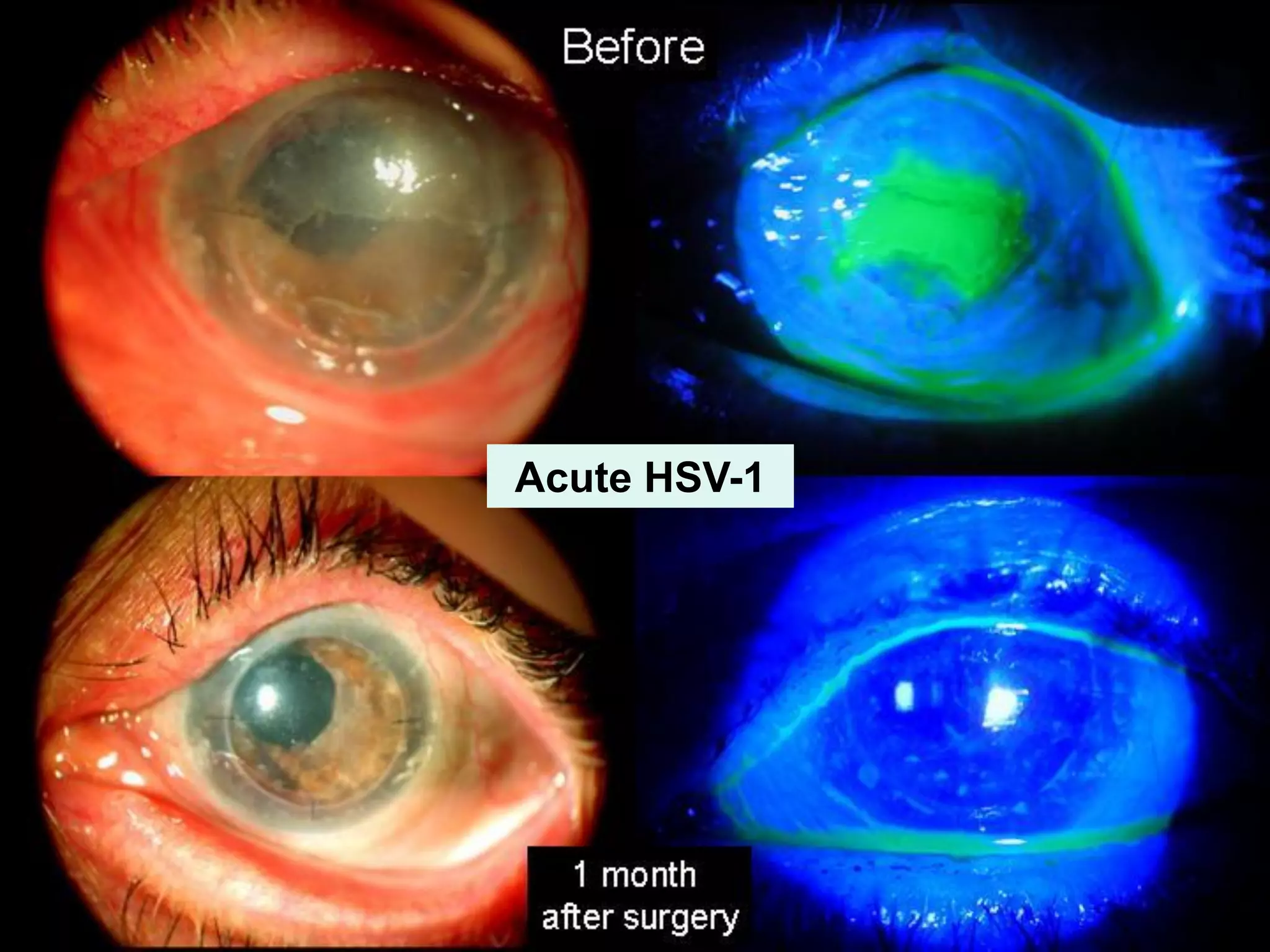
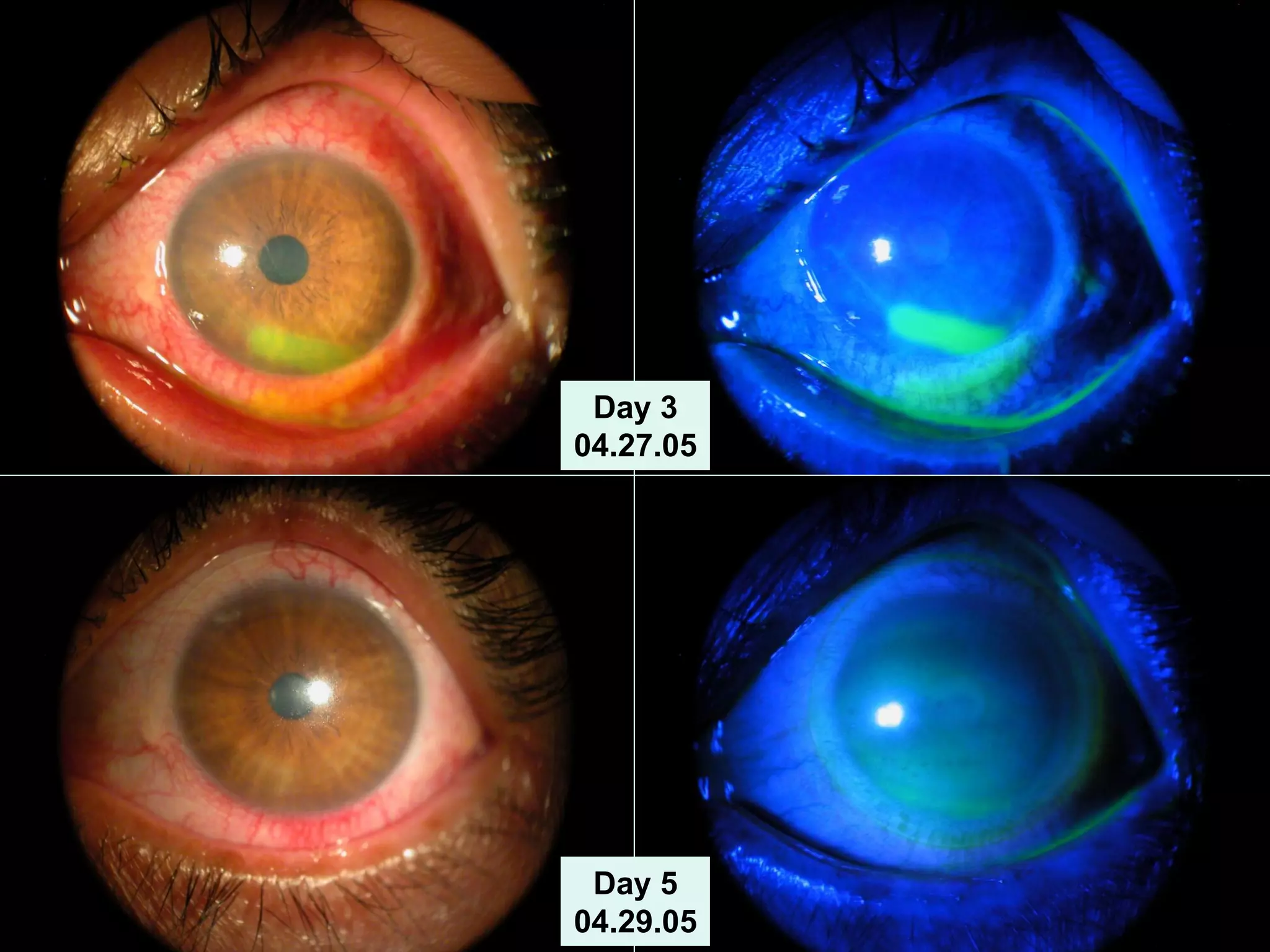
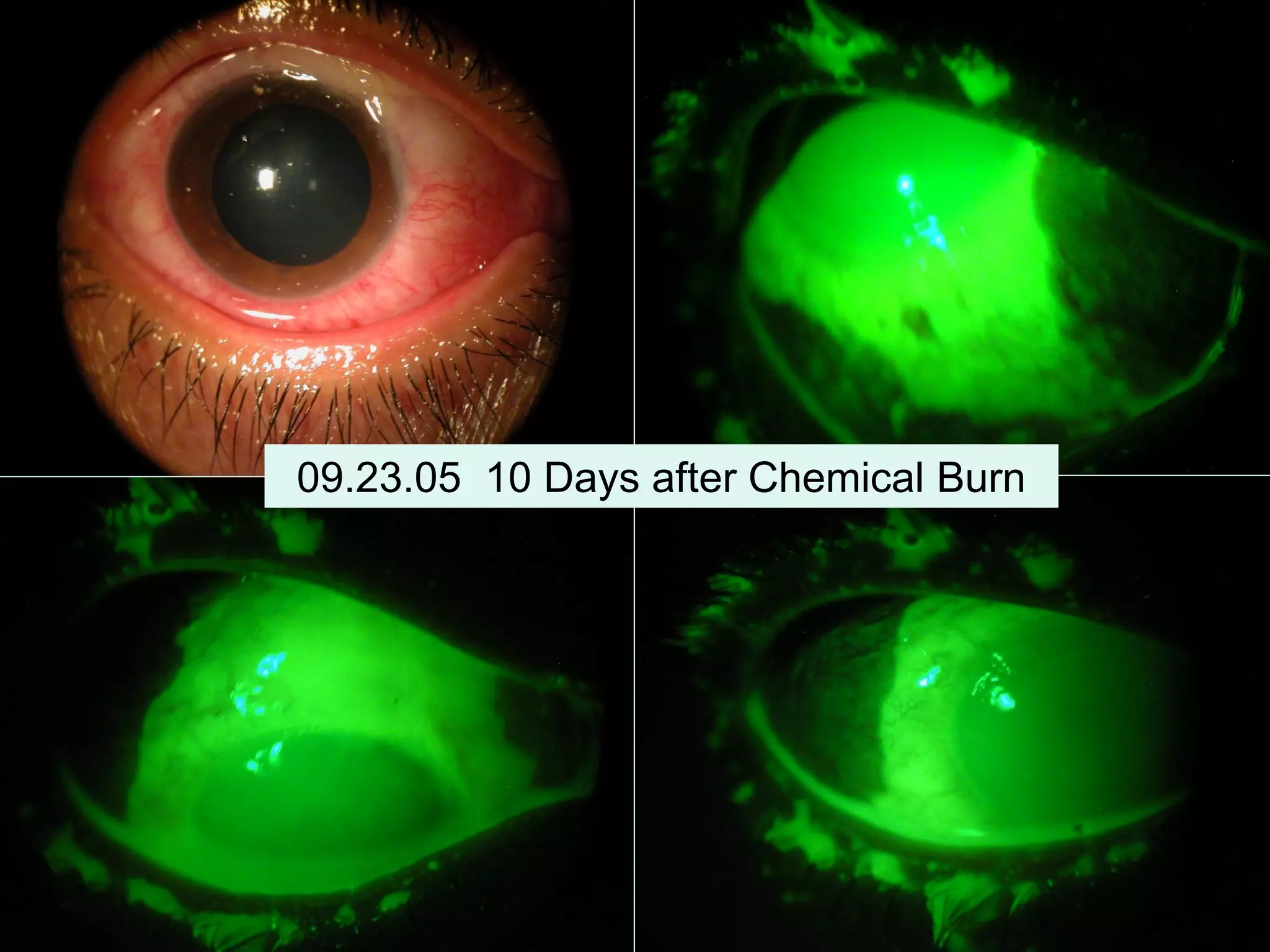
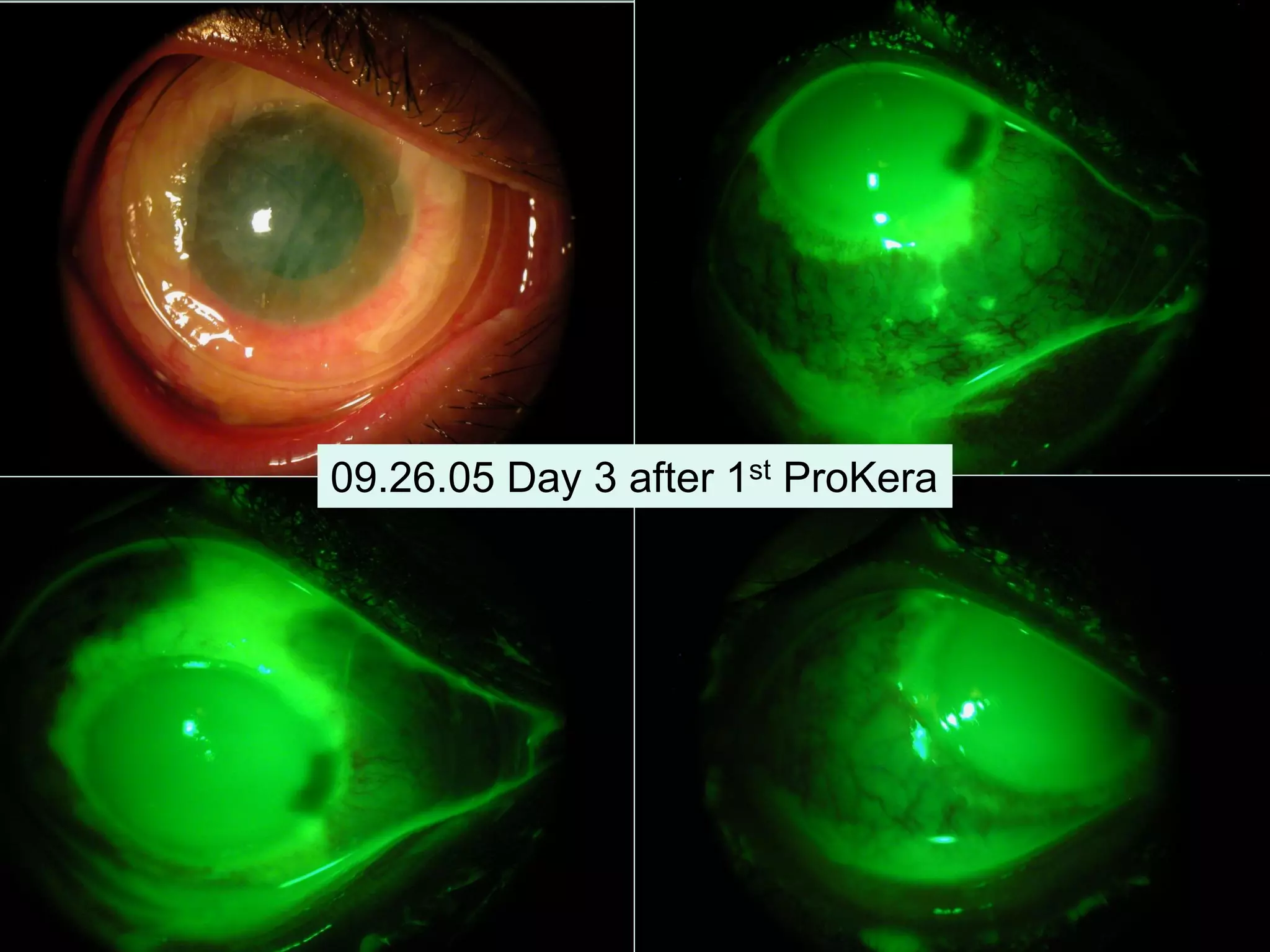
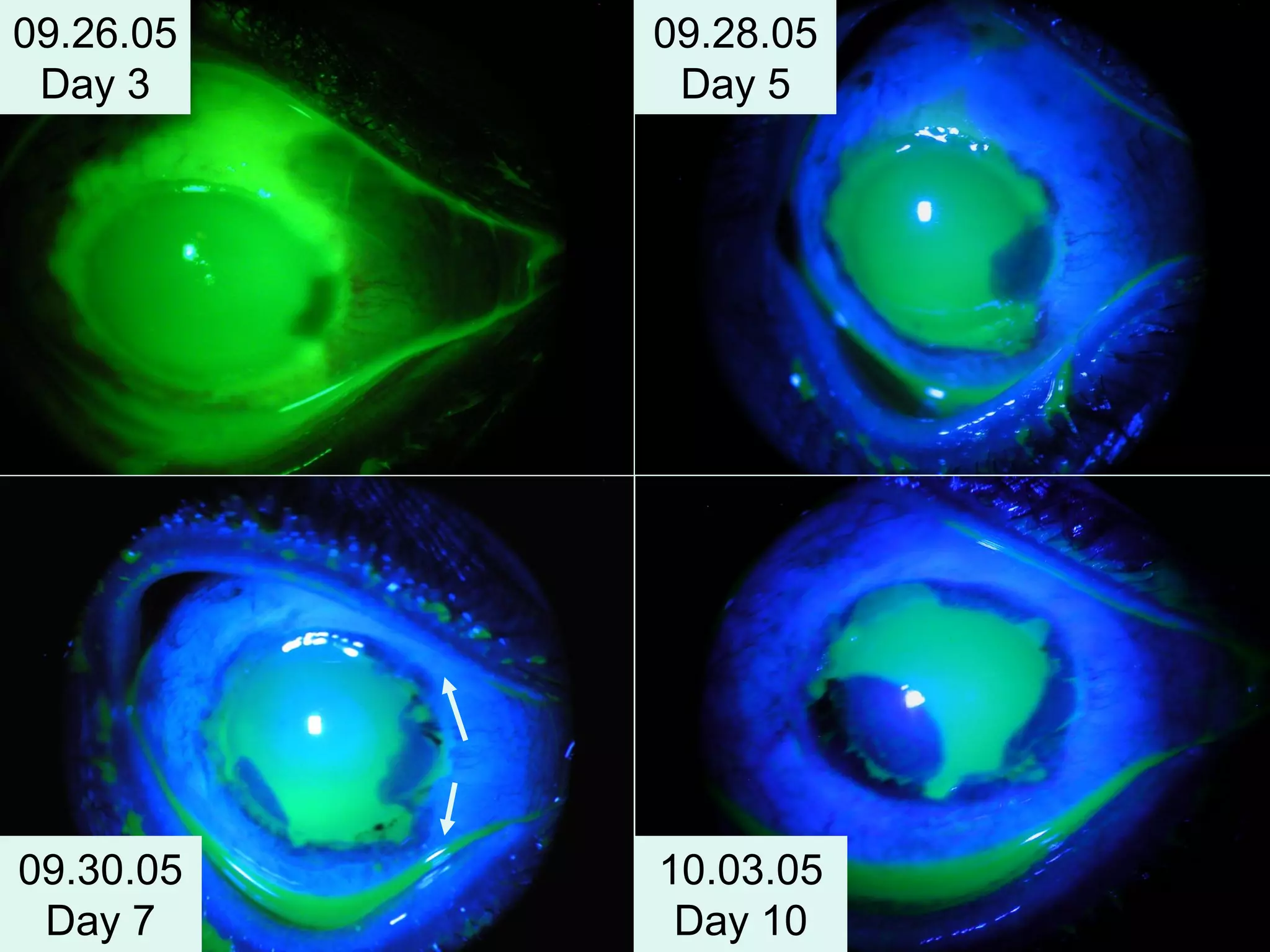
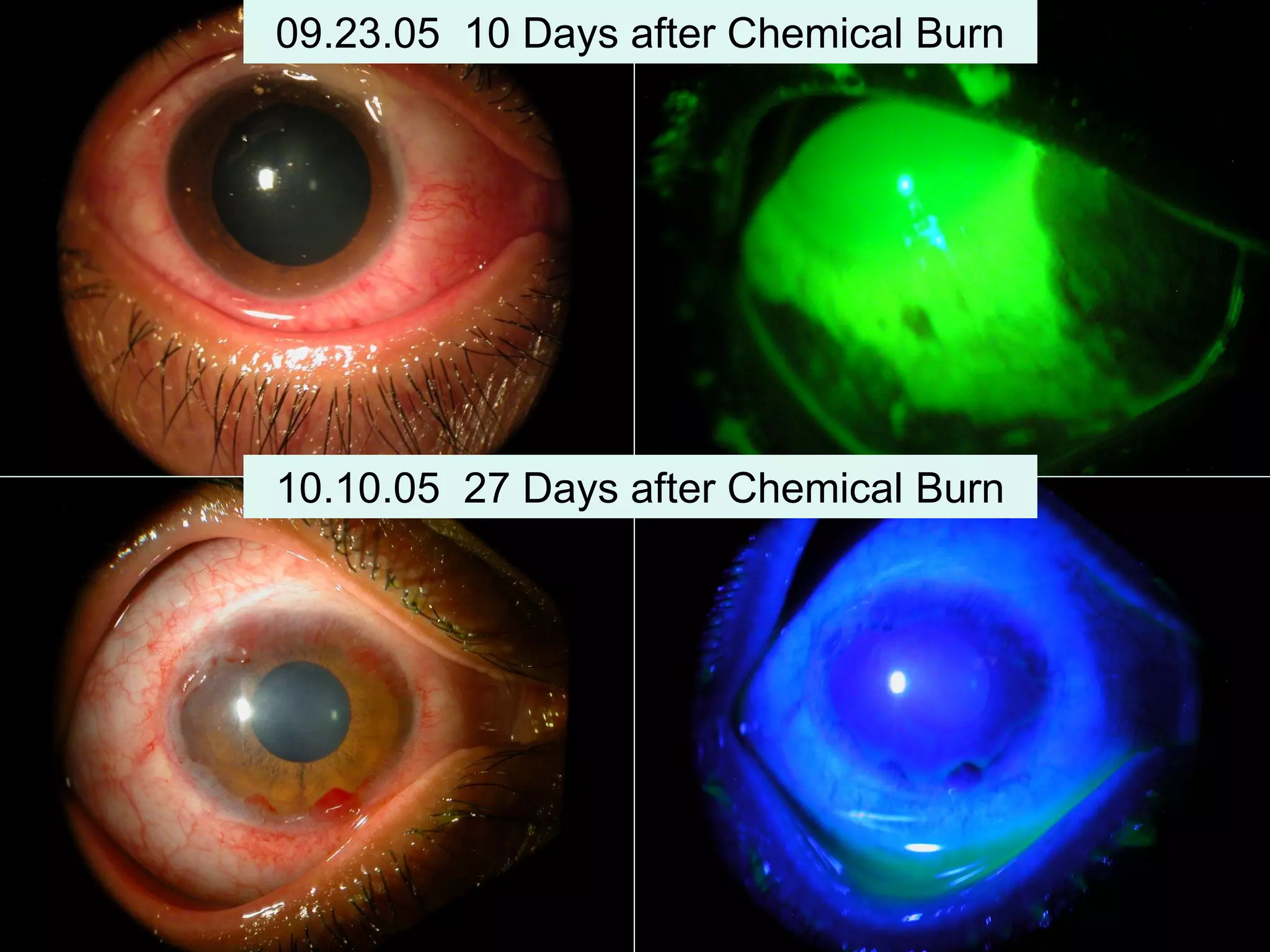
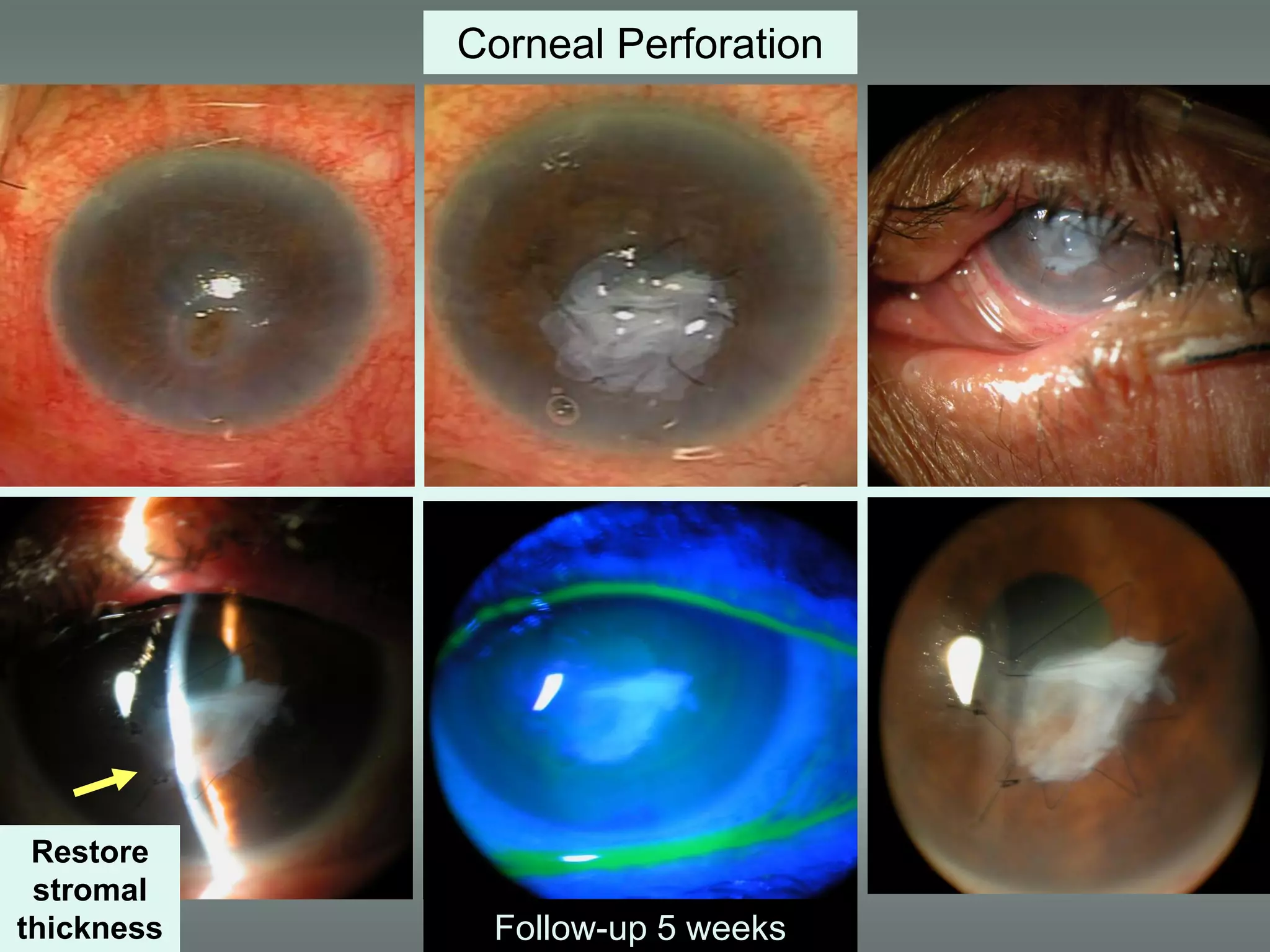
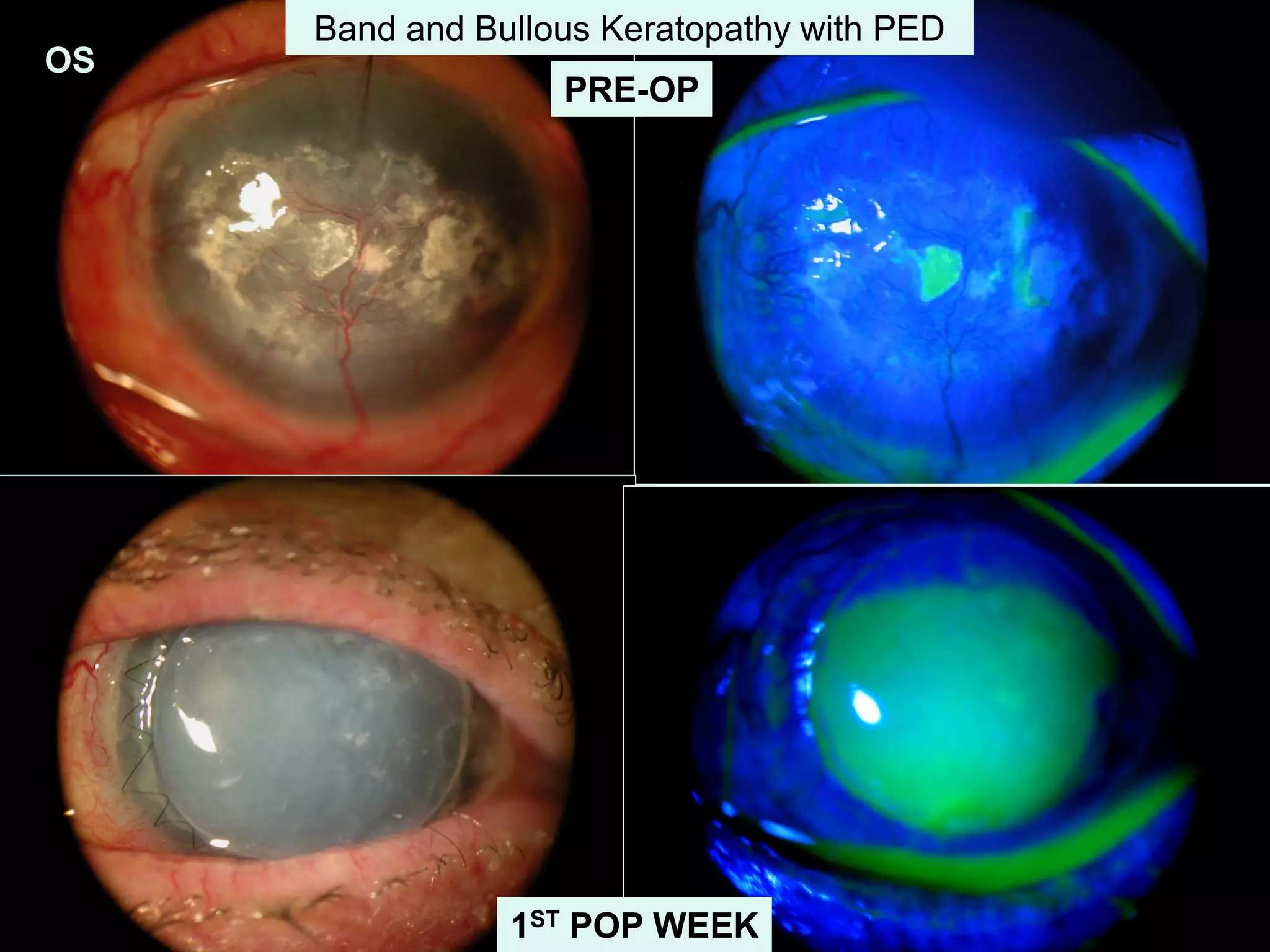
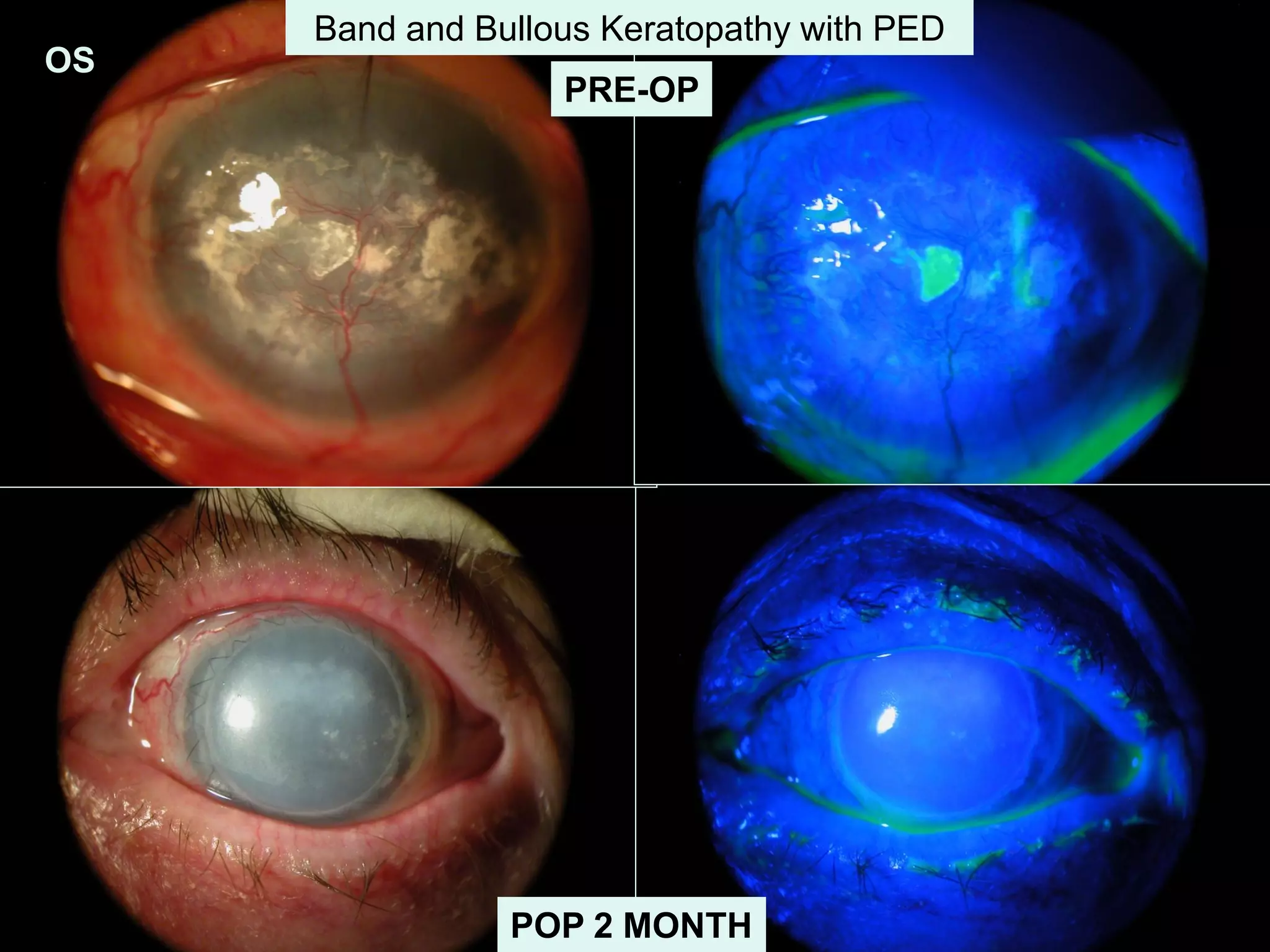
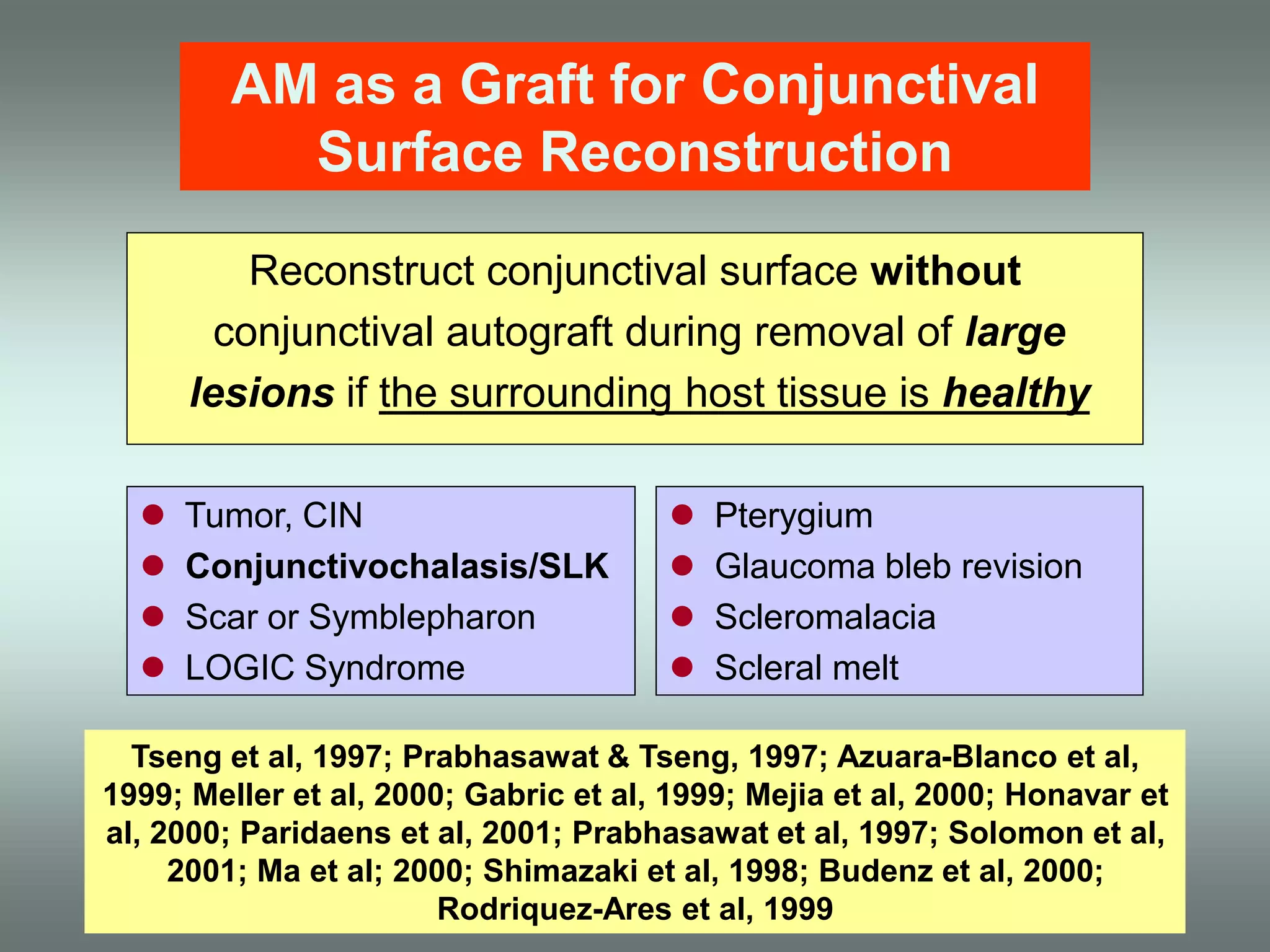
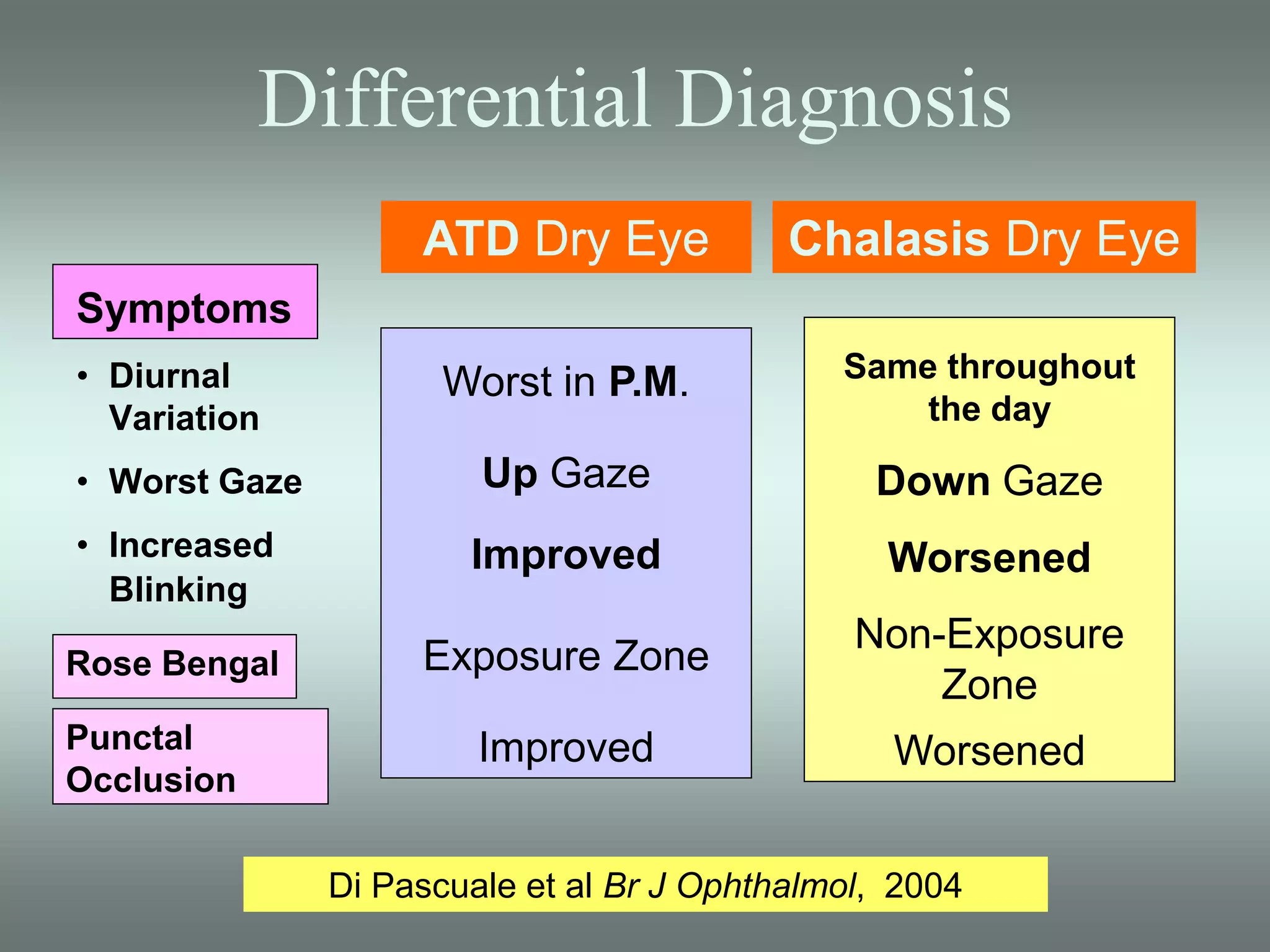
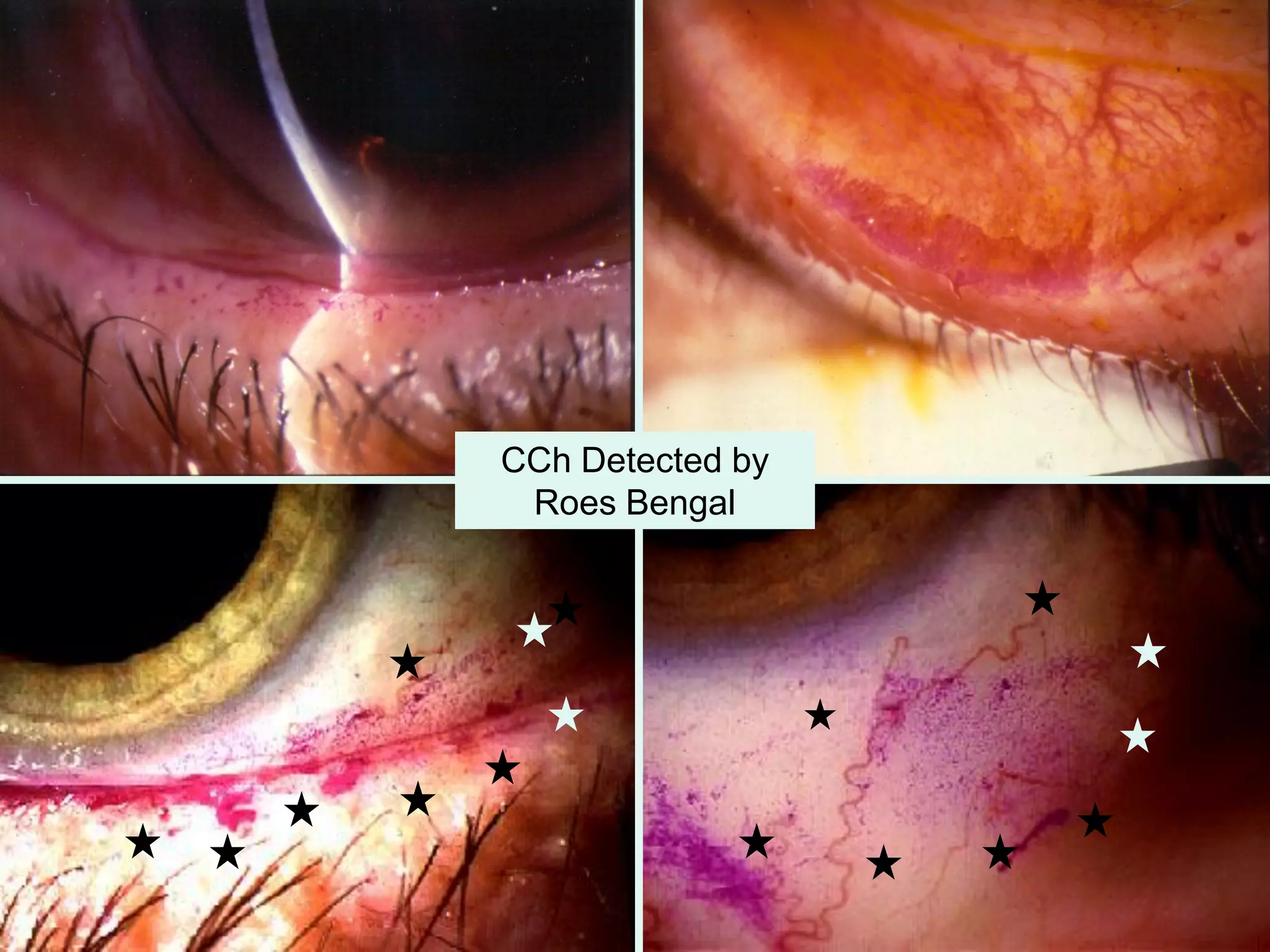
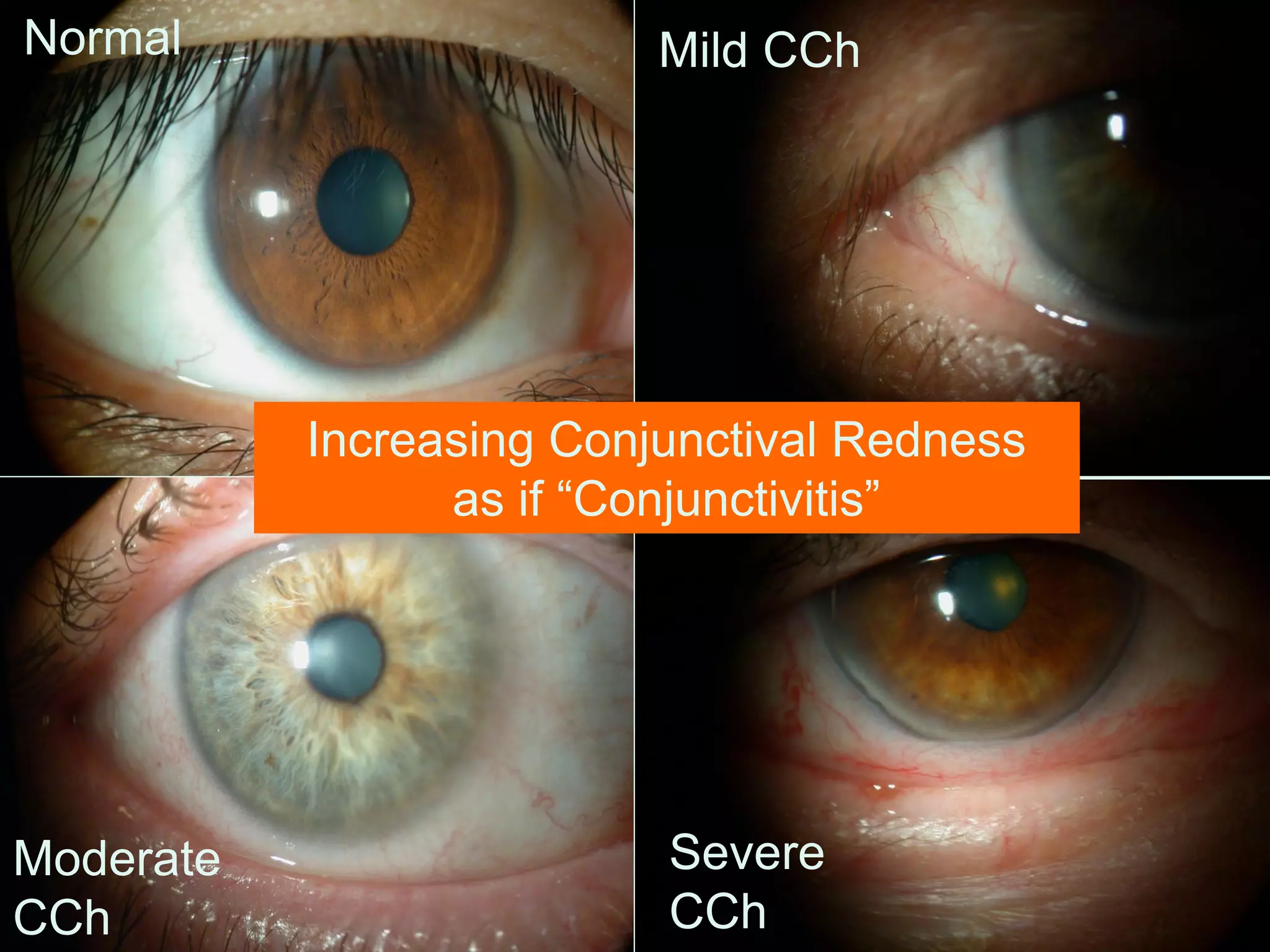
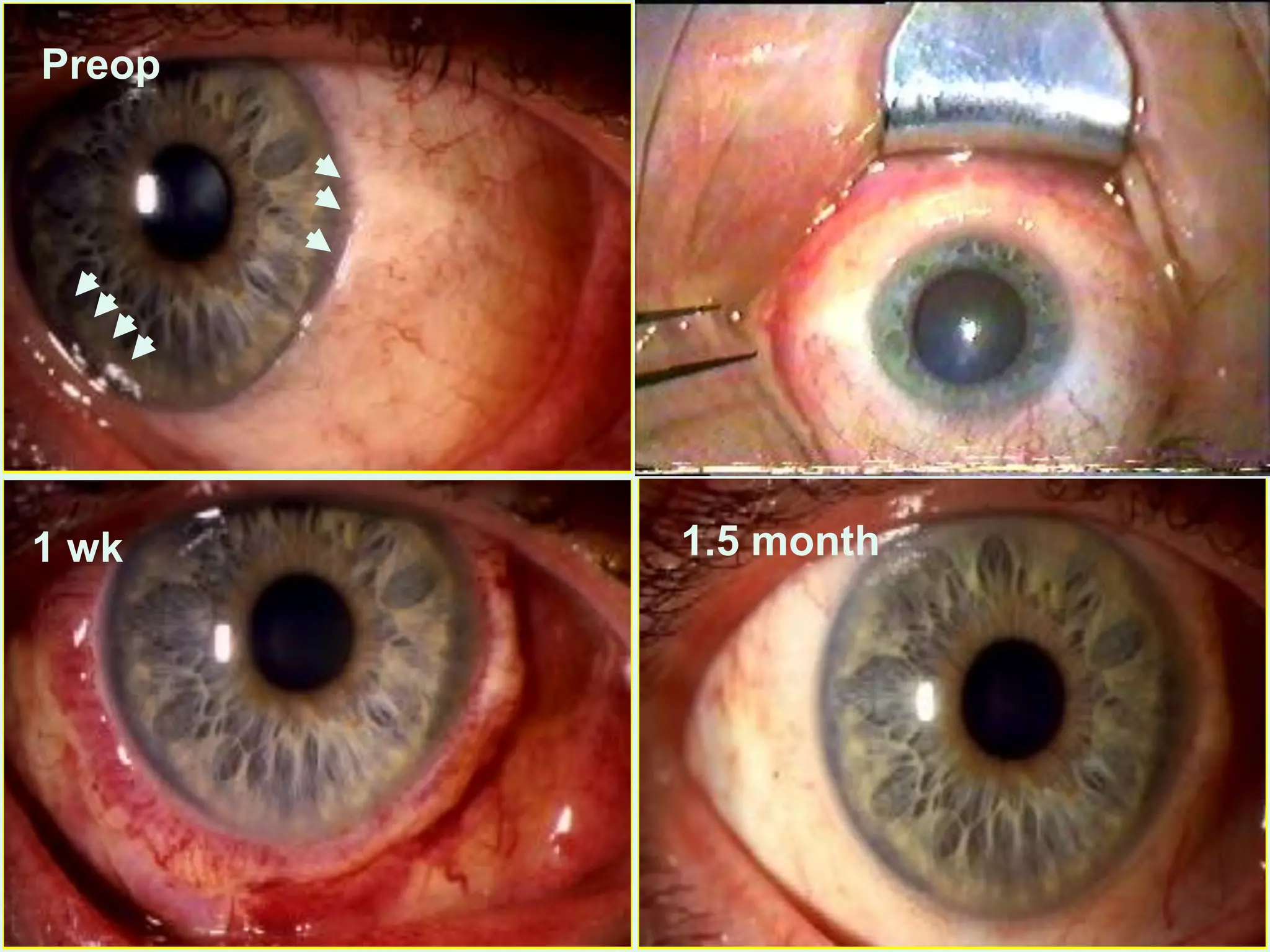
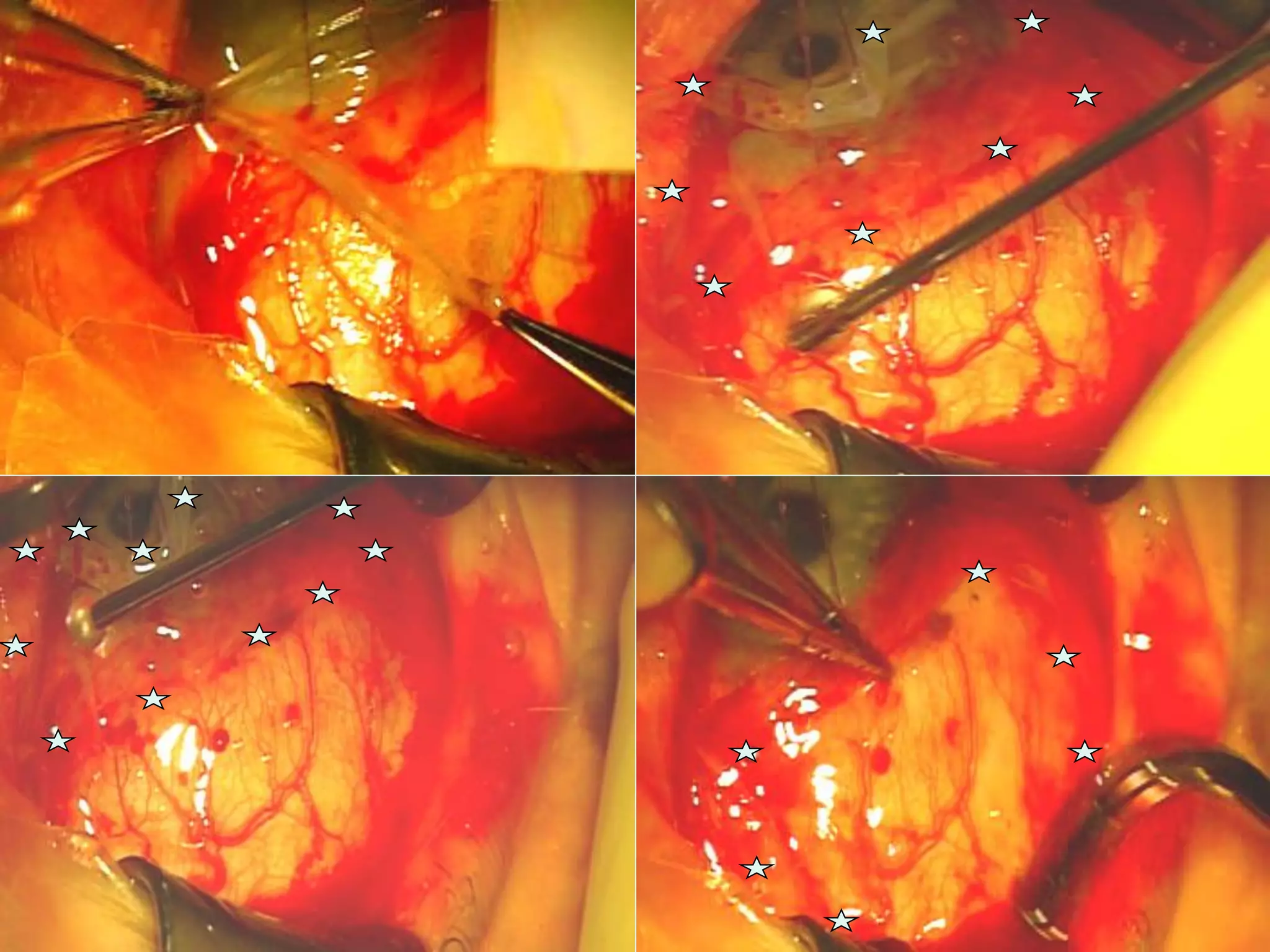
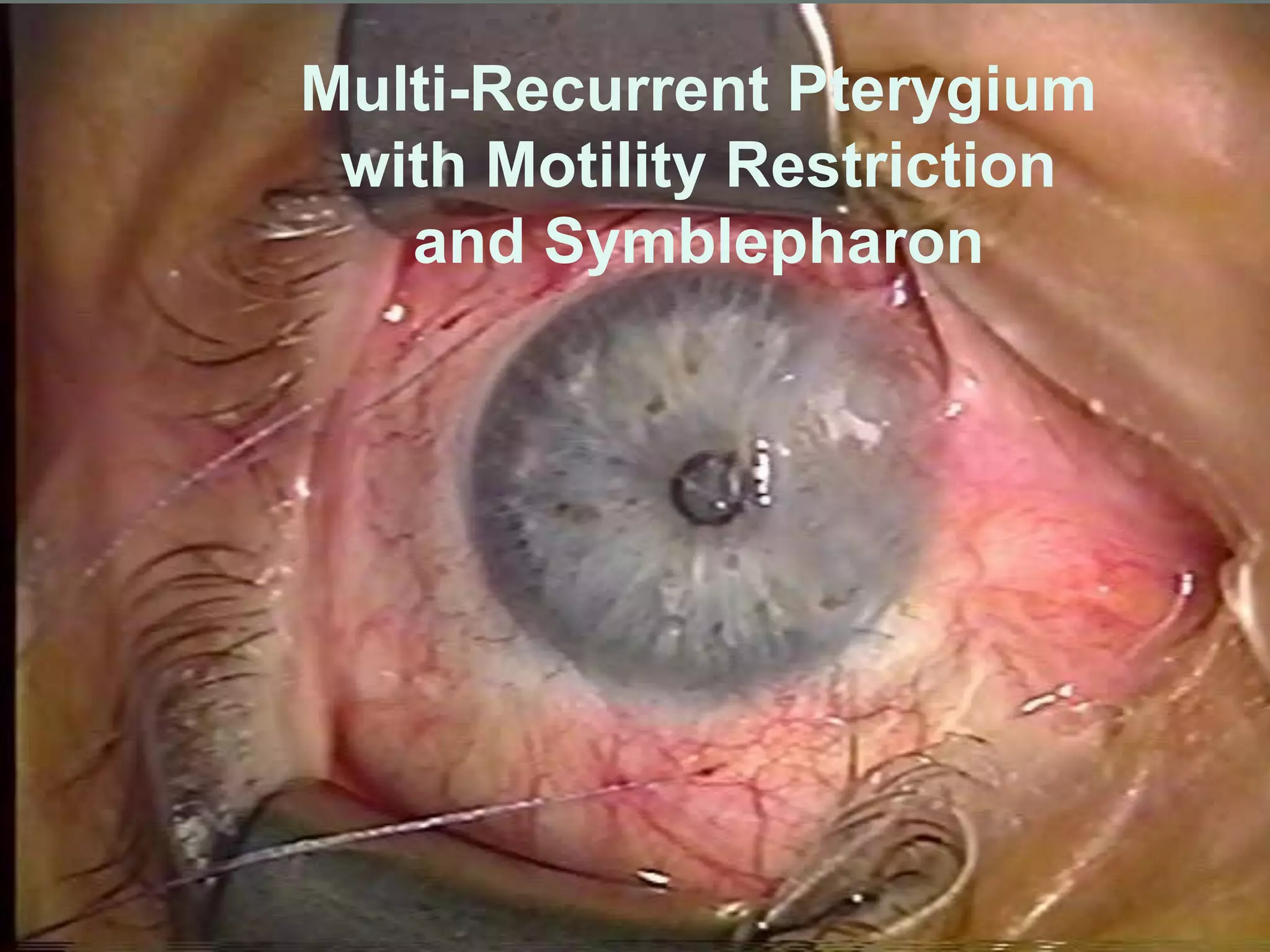
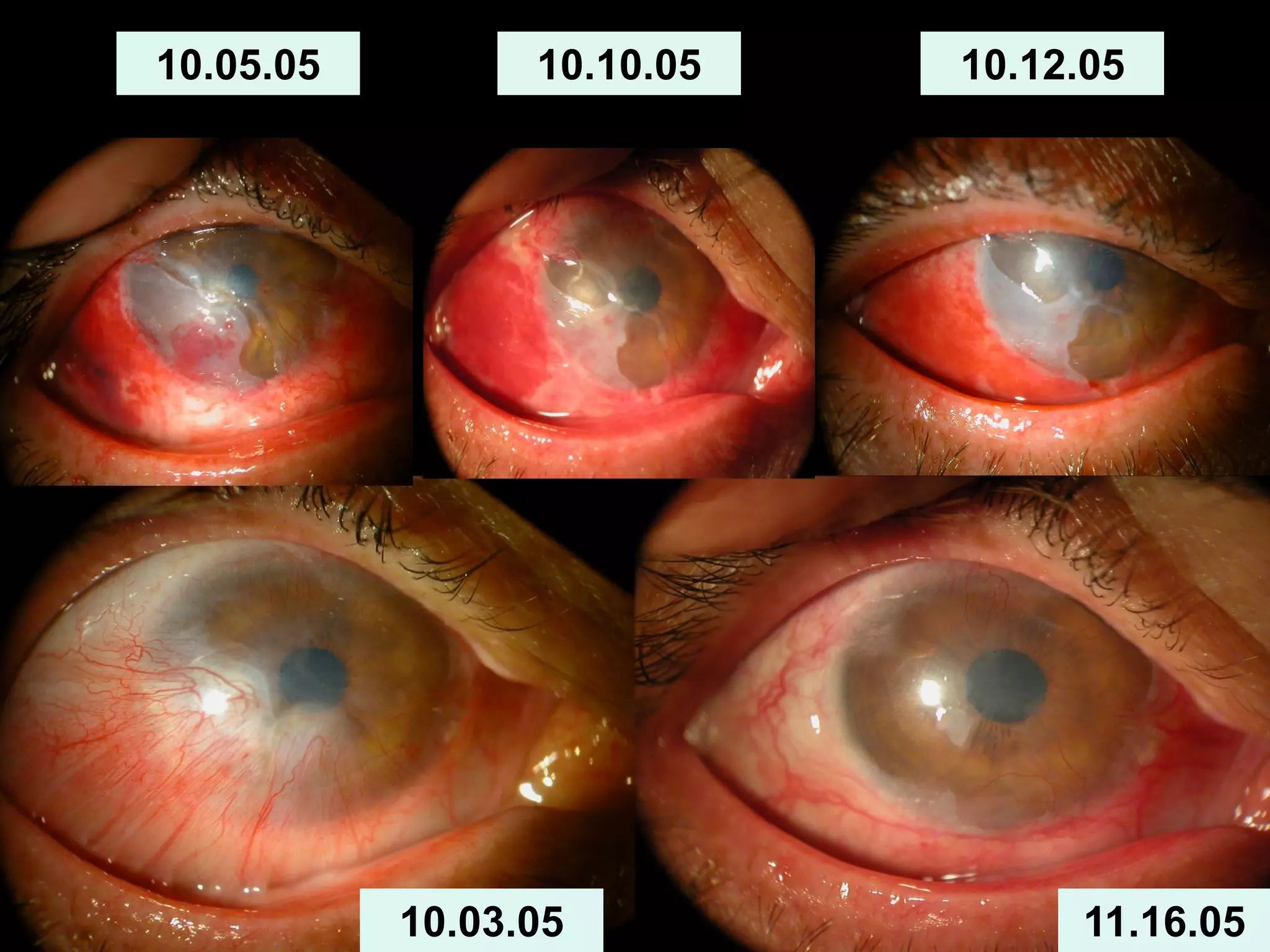
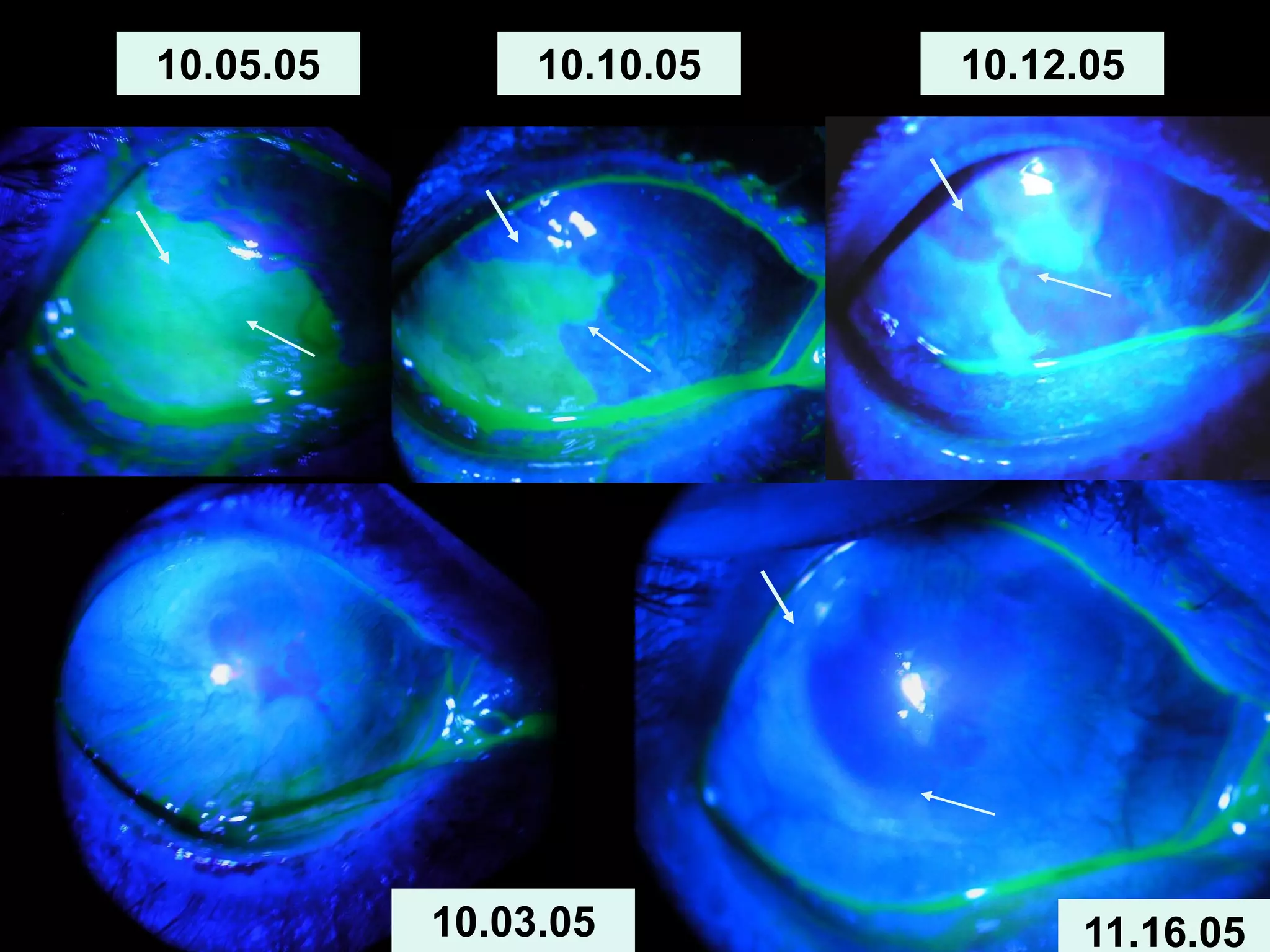
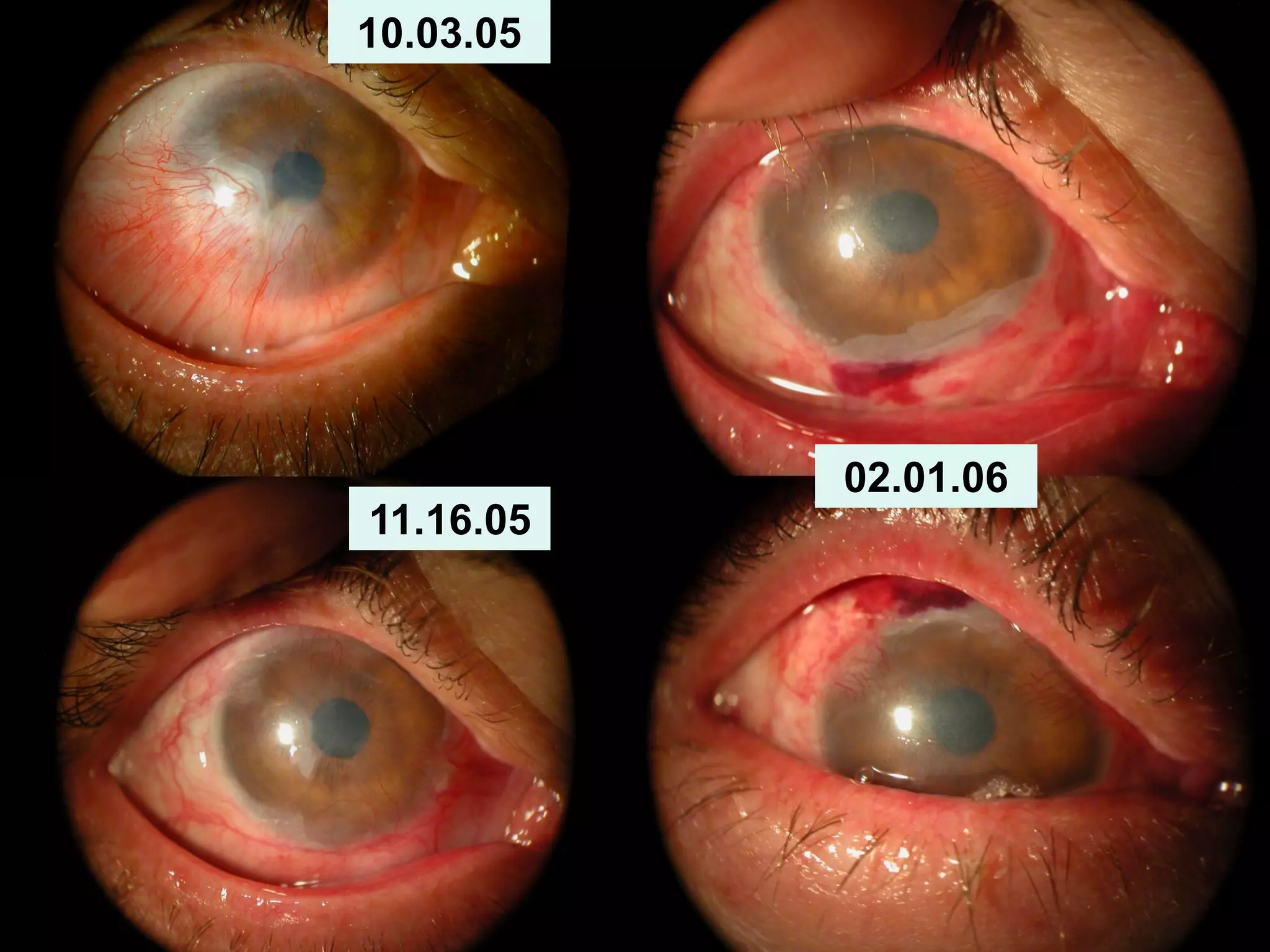
The document discusses the sutureless approach of amniotic membrane transplantation (AMT) using cryopreserved amniotic membrane (amniograft®), which is FDA approved for ocular surface reconstruction. It outlines various indications for AMT, its applications in treating conditions like chemical burns and conjunctivochalasis, and how it promotes healing and reduces inflammation and scarring. The document emphasizes the use of amniograft® as both temporary and permanent grafts, supported by clinical data and research findings.