The document presents a project report on designing a plant to manufacture 50,000 tons per year of styrene oxide. Key points:
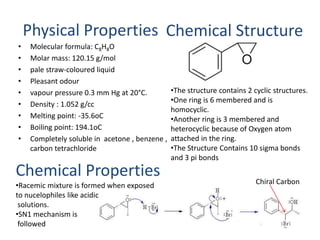
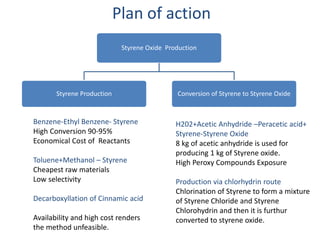
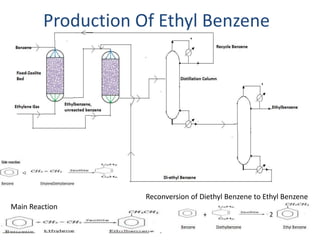
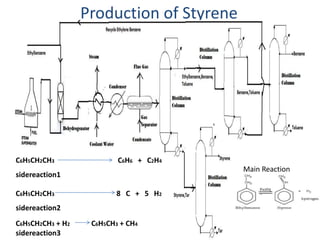
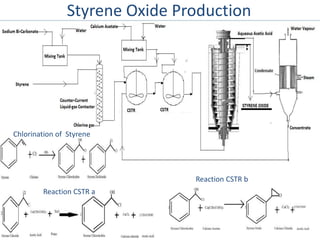
1) Styrene oxide is produced through the oxidation of styrene and will be manufactured using a process involving the production of ethylbenzene, styrene, and then chlorination of styrene to produce styrene oxide.
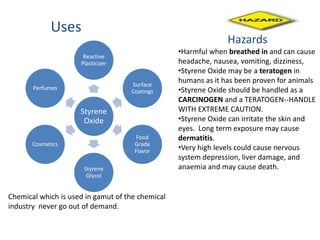
2) Safety and environmental hazards around styrene oxide production will need to be addressed as it is a potential carcinogen and teratogen.
3) The designed plant will include equipment like continuous stirred-tank reactors to perform the necessary chemical reactions.