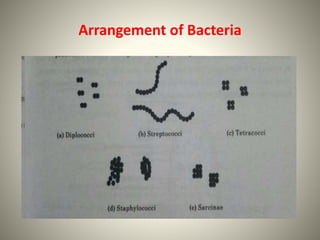
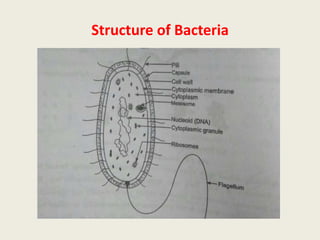
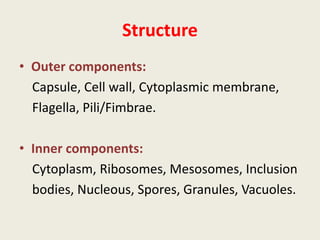
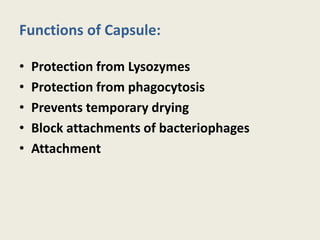
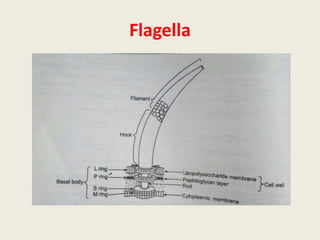
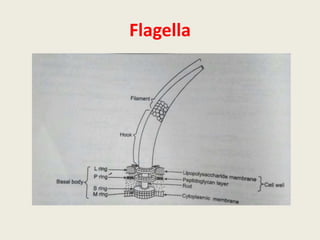
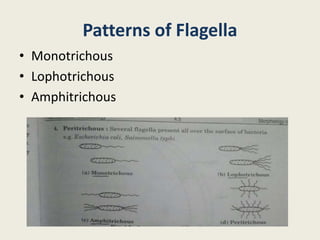
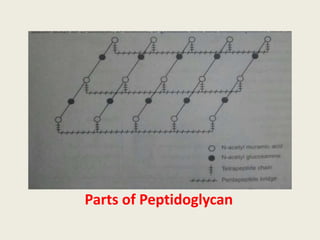
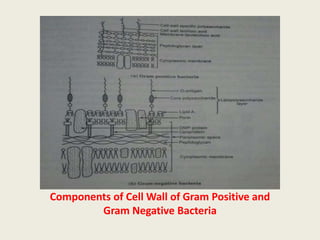
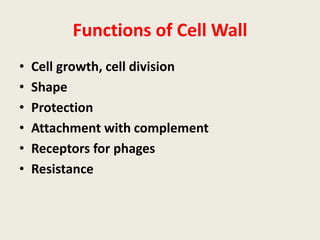
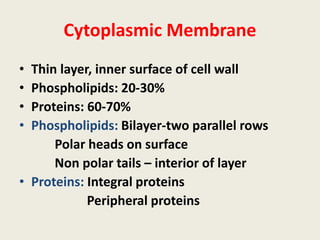
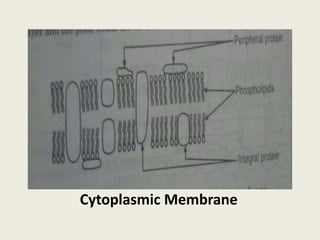
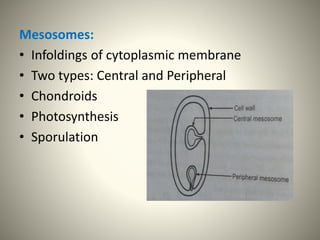
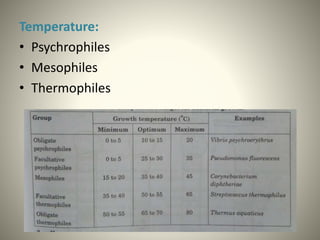
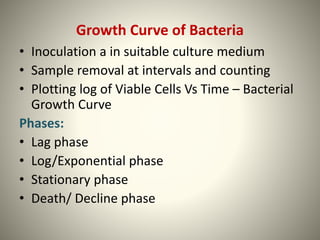
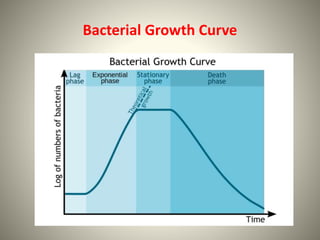
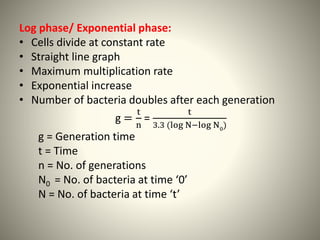
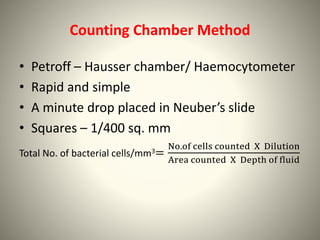
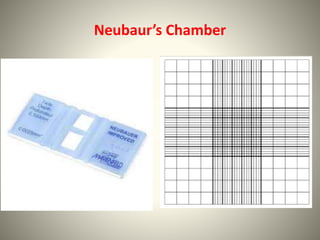
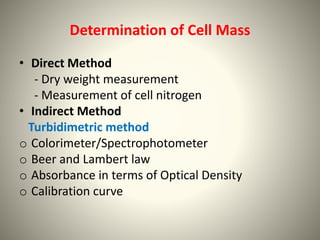
The document provides a comprehensive overview of bacterial structure, including their outer (capsule, cell wall, cytoplasmic membrane, flagella, pili) and inner components (cytoplasm, ribosomes, nucleus, spores). It also discusses bacterial nutritional requirements, growth curves, and methods for measuring and preserving bacterial cultures, highlighting the differences between gram-positive and gram-negative bacteria. Additionally, it enumerates biochemical tests used for bacterial identification and metabolic characterization.