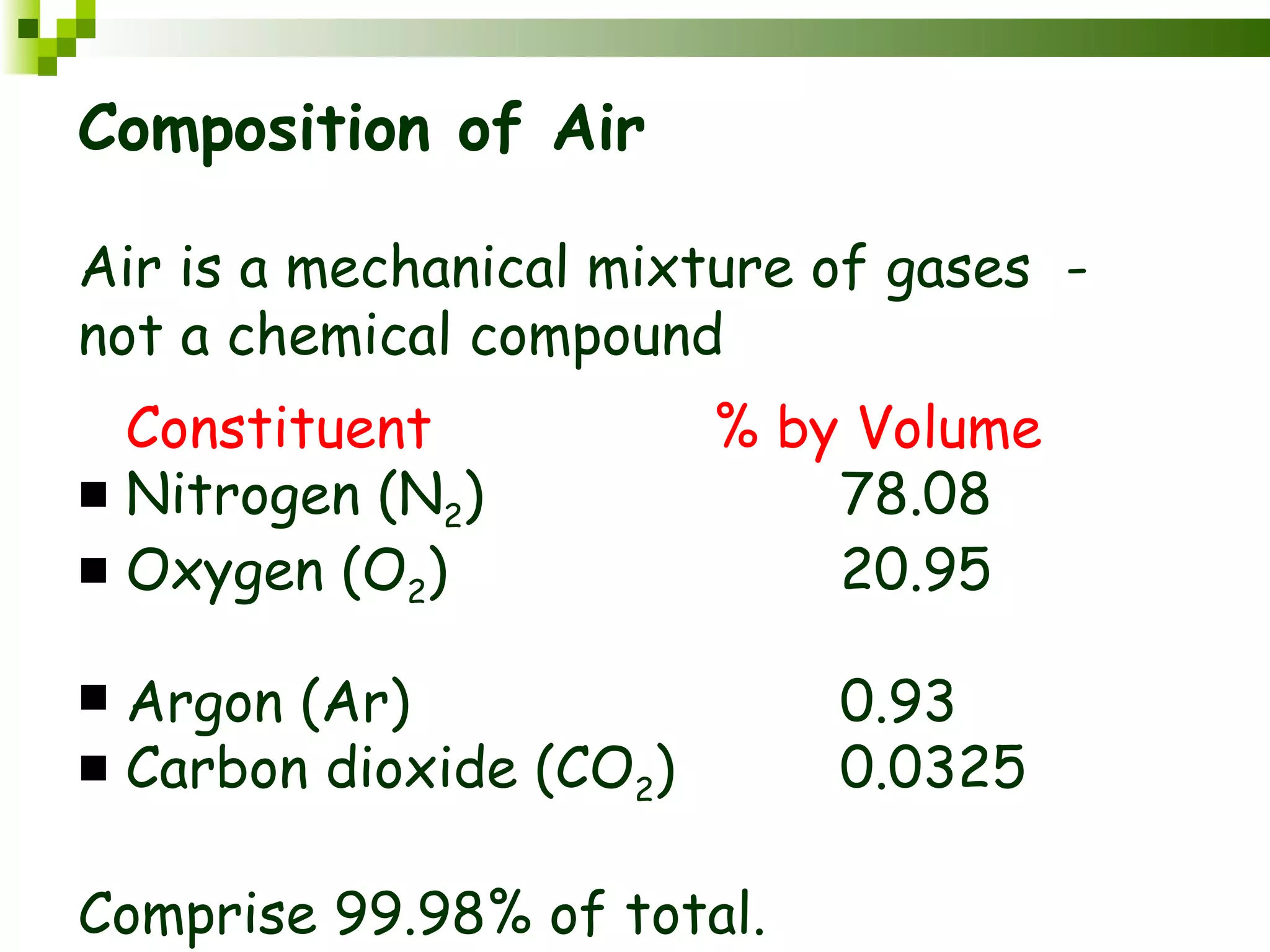
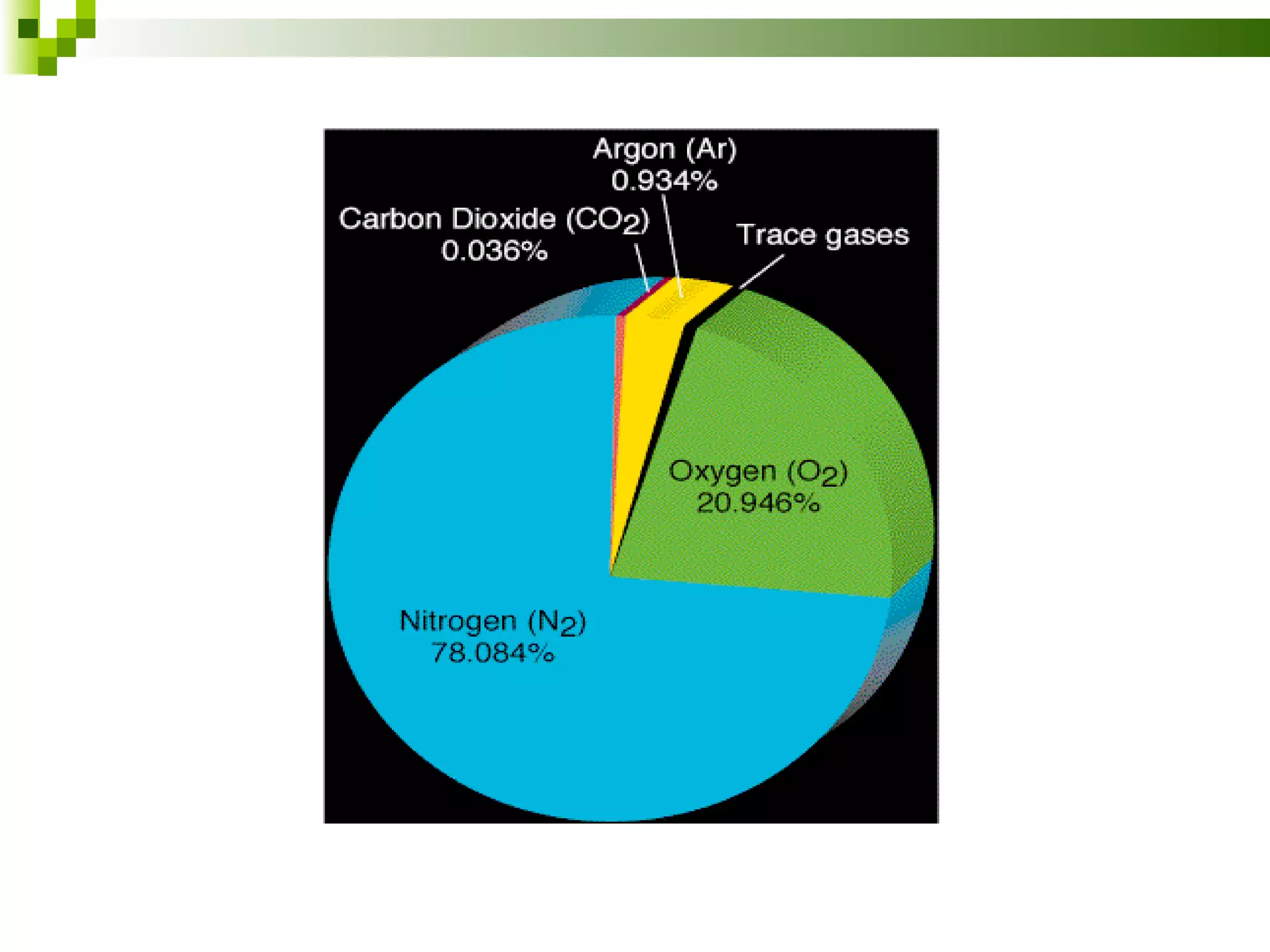
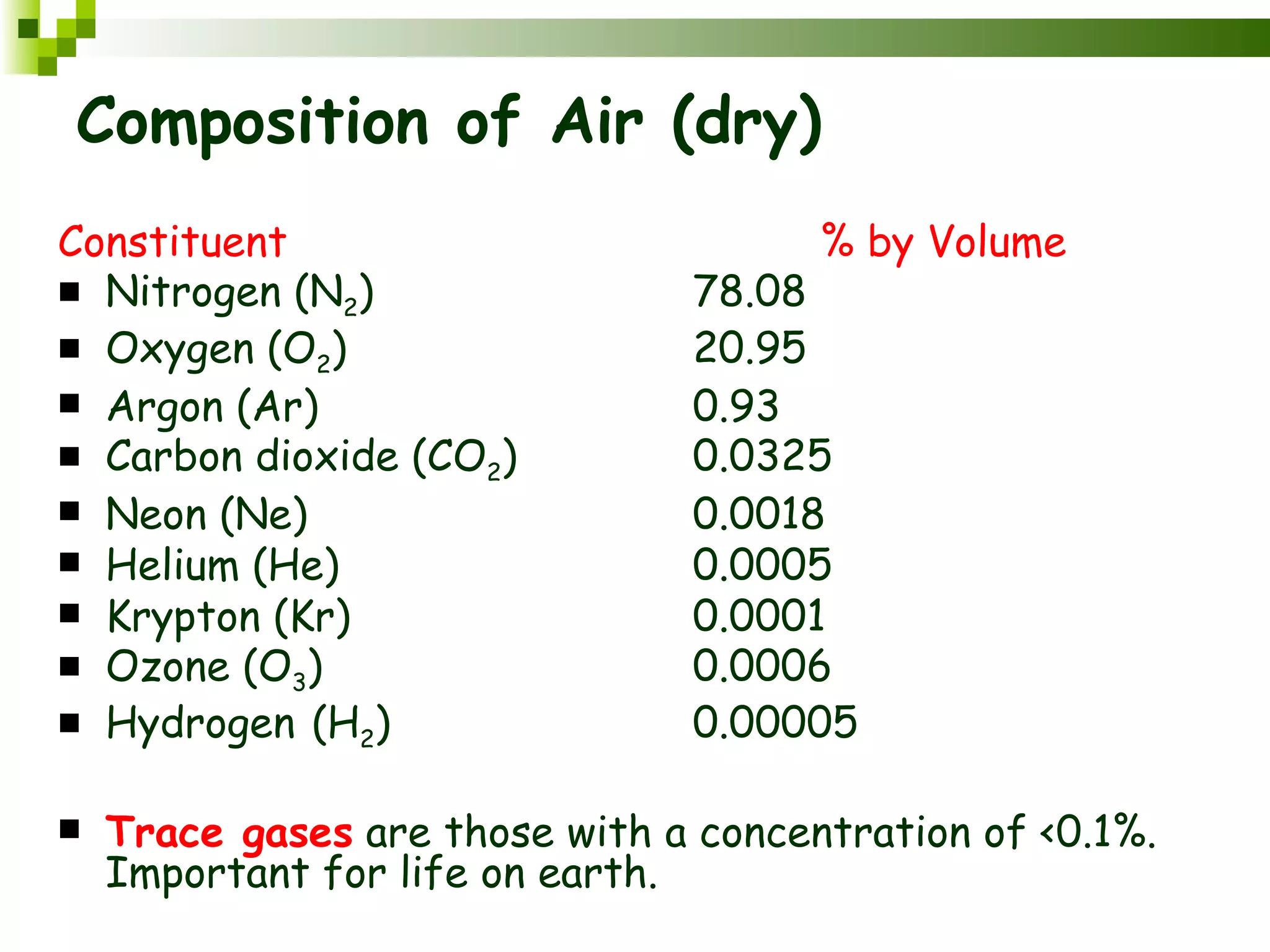
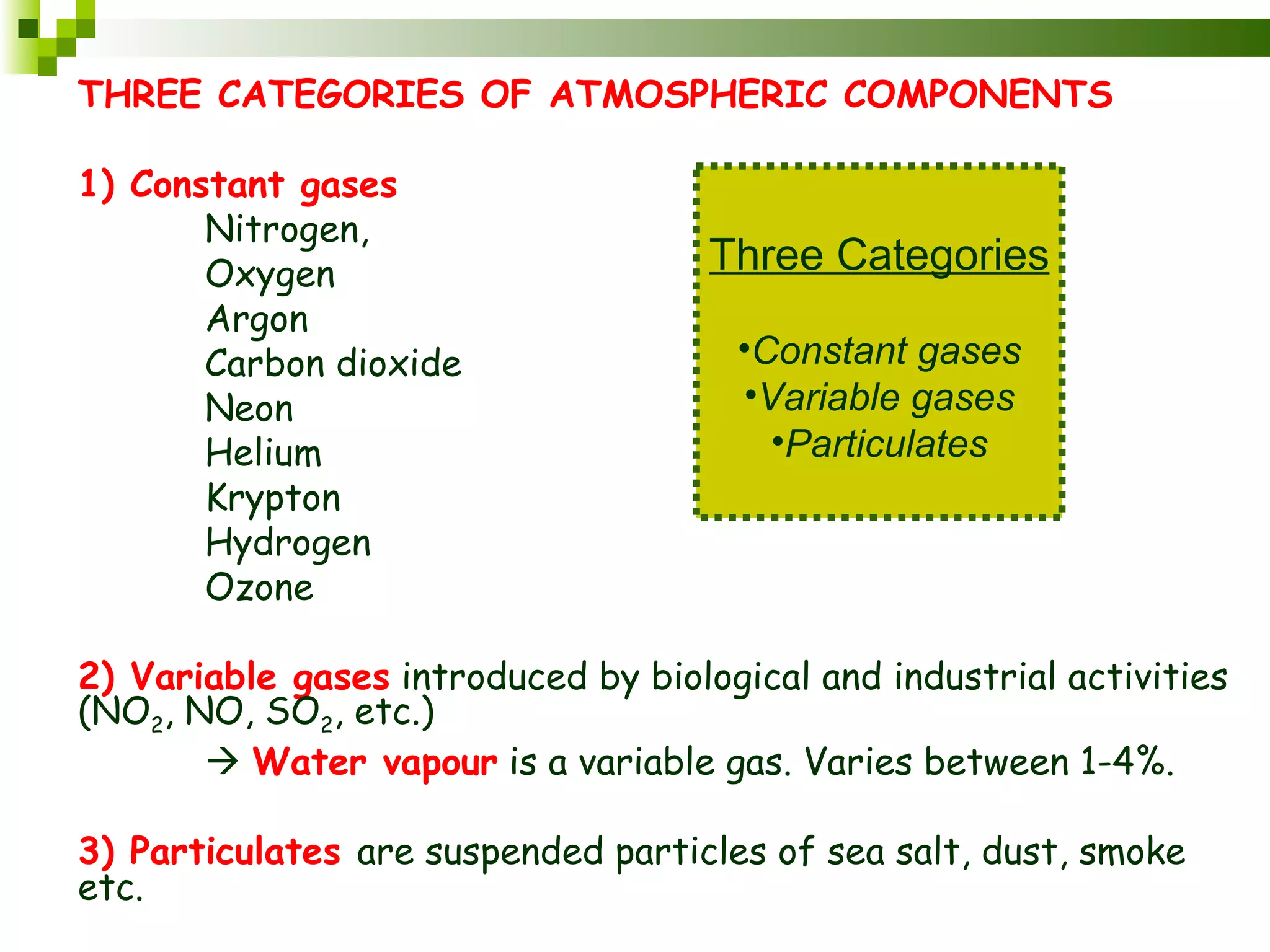
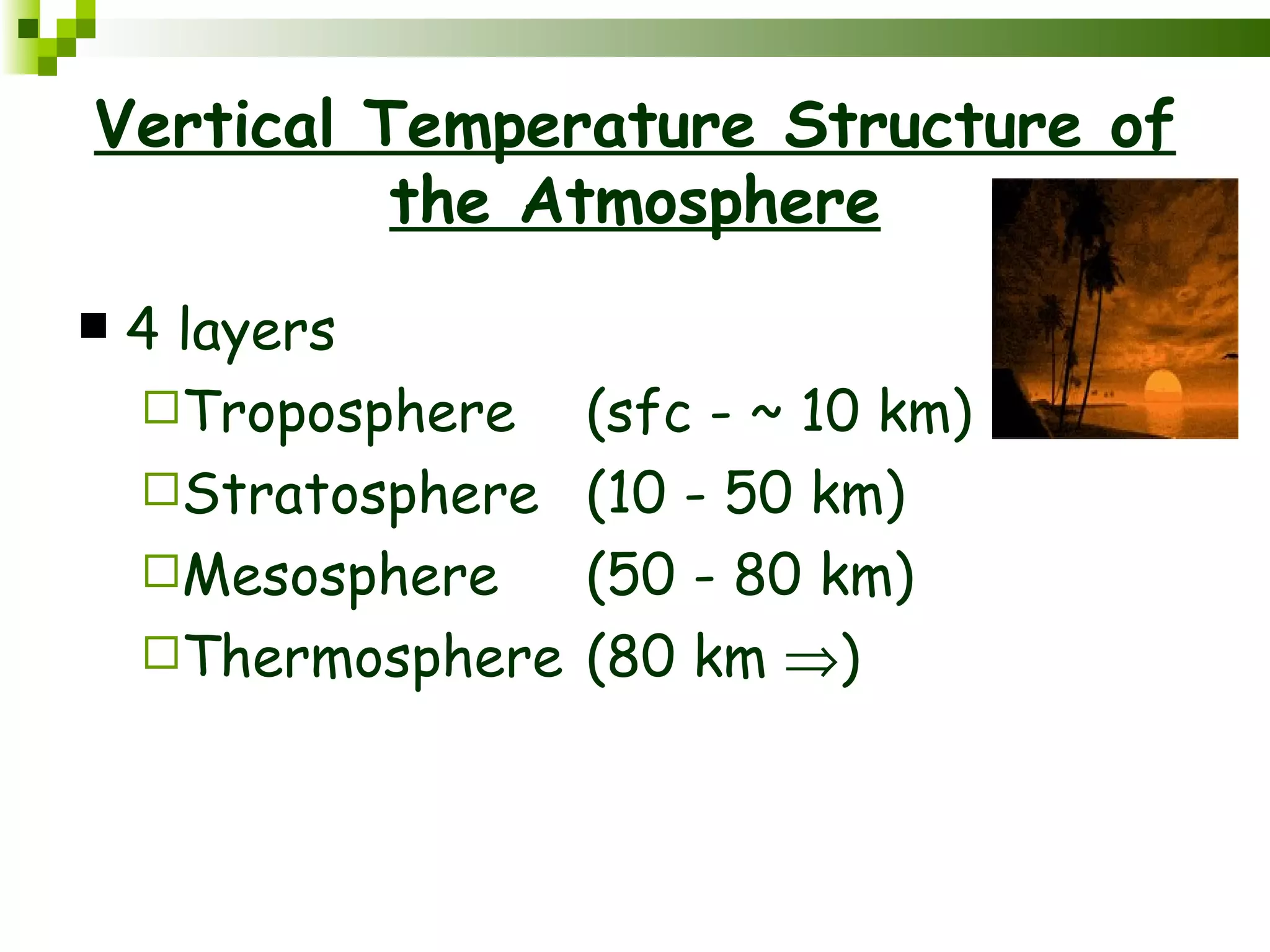
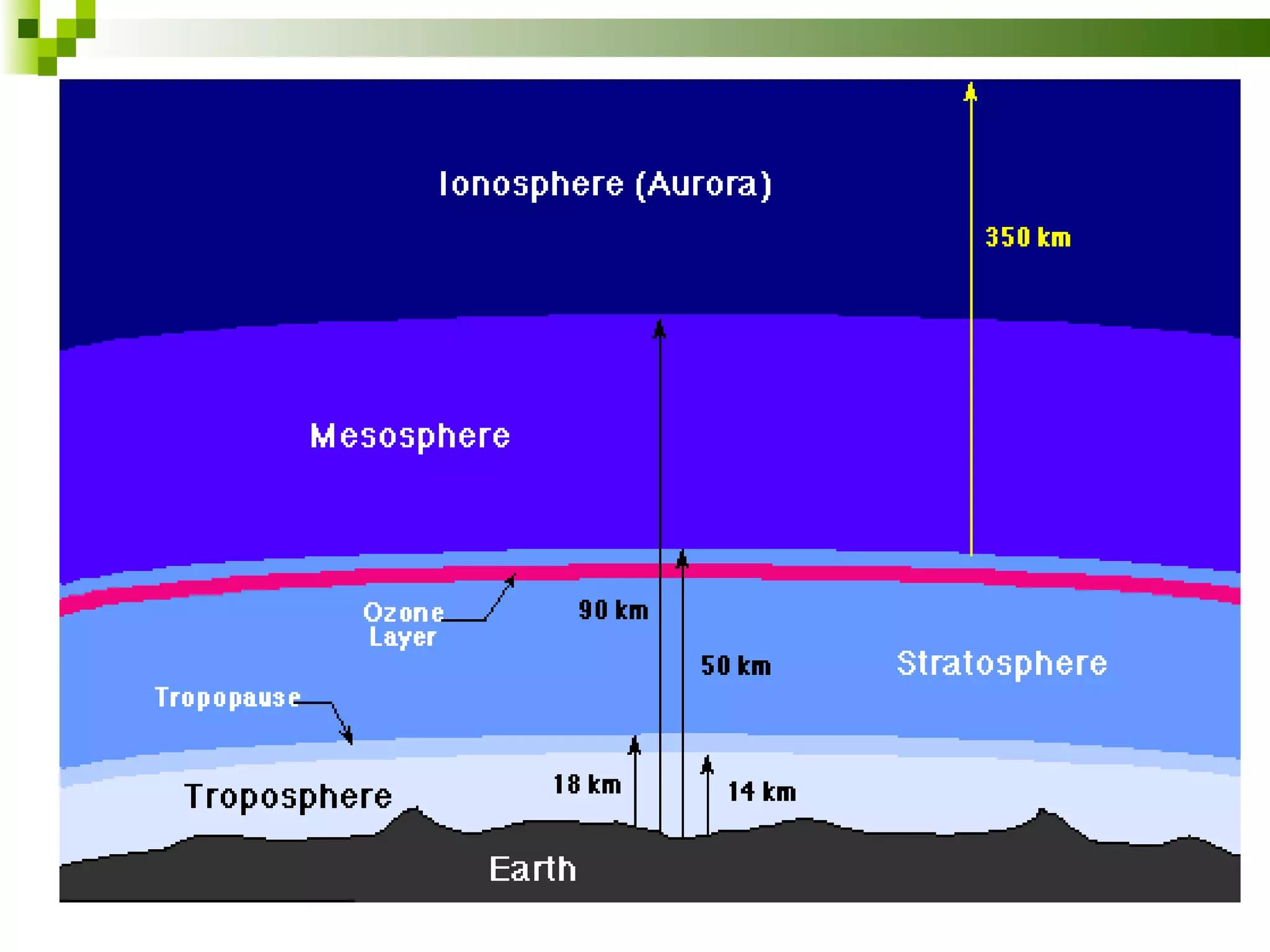
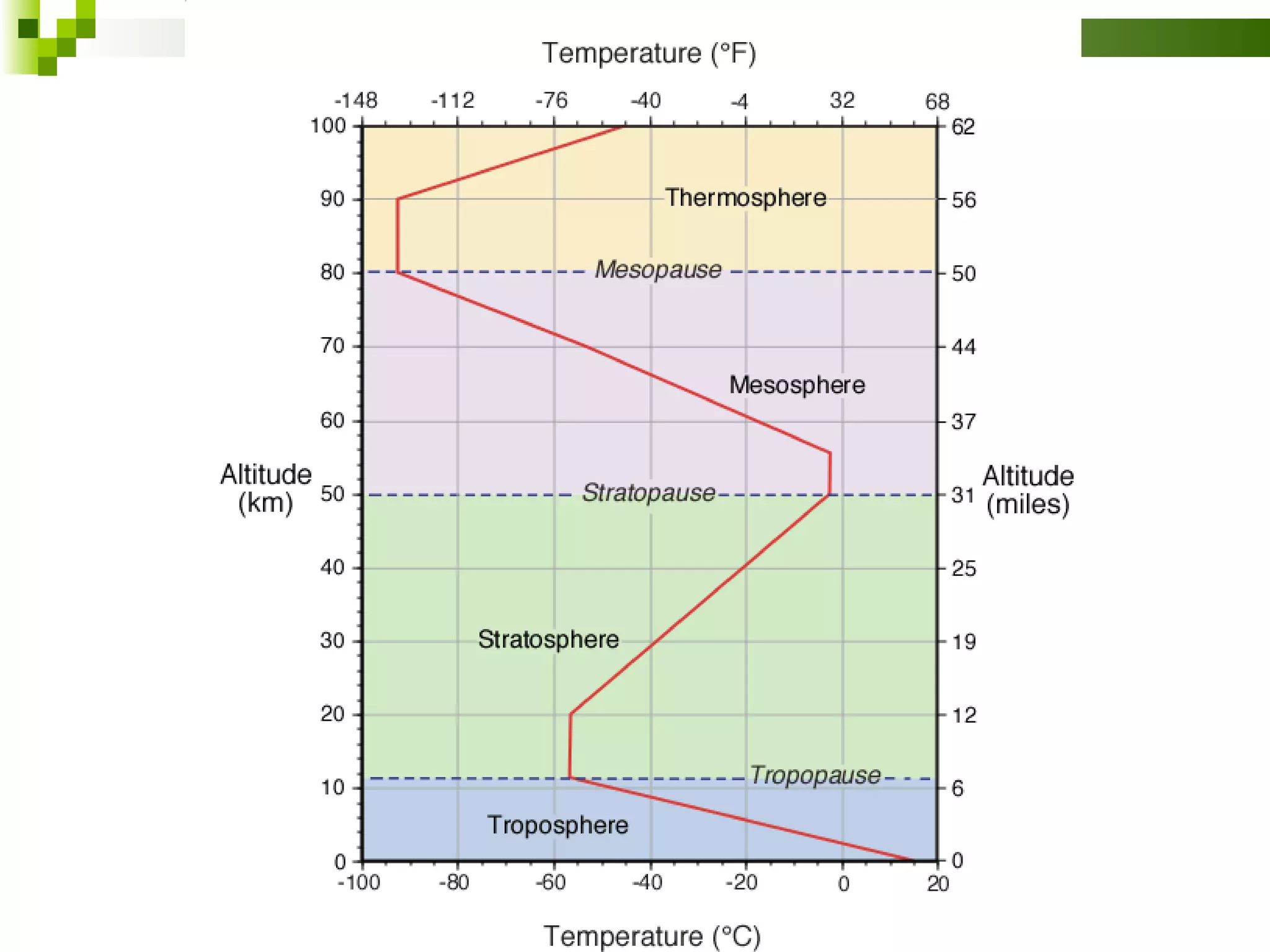
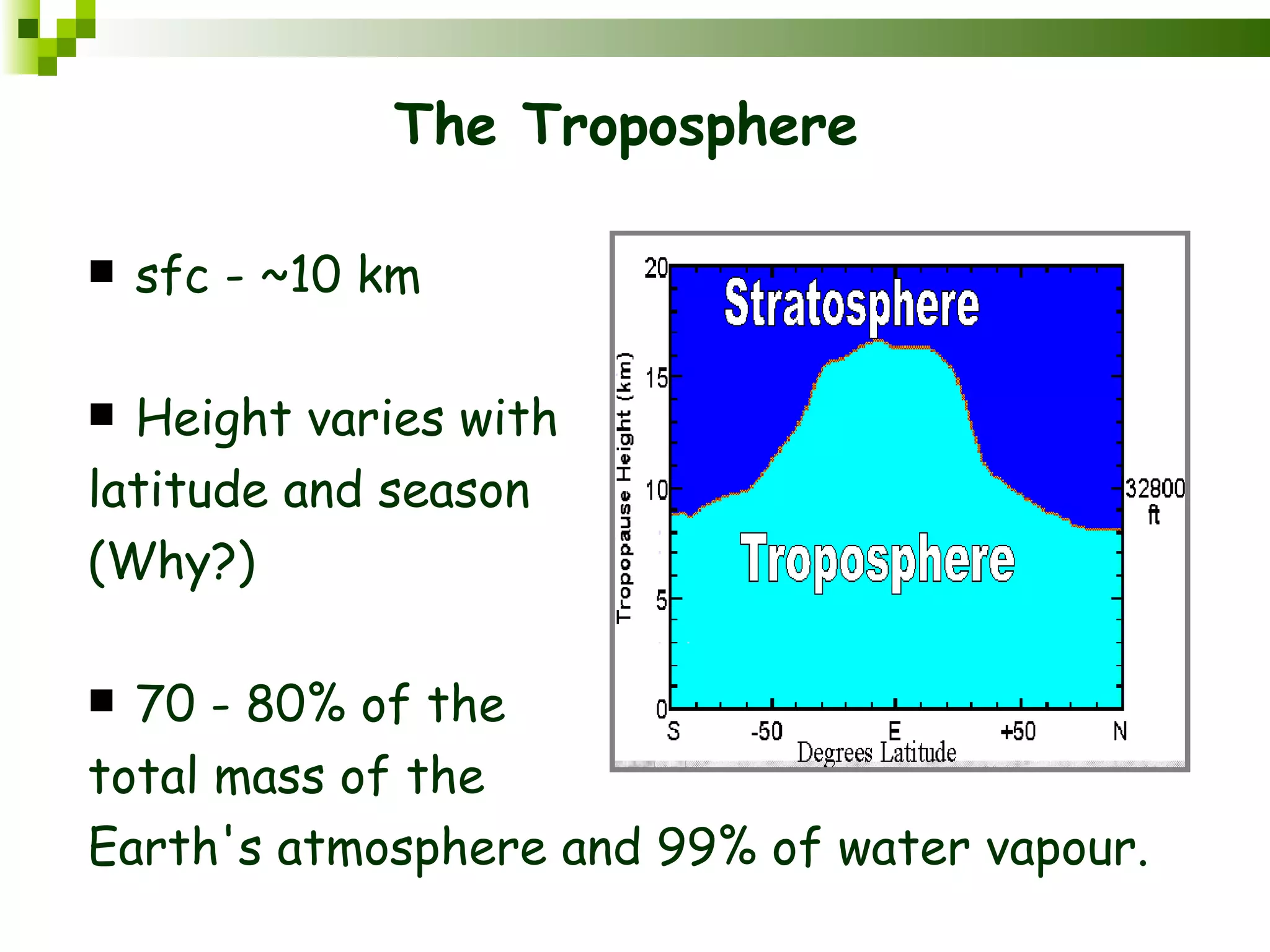
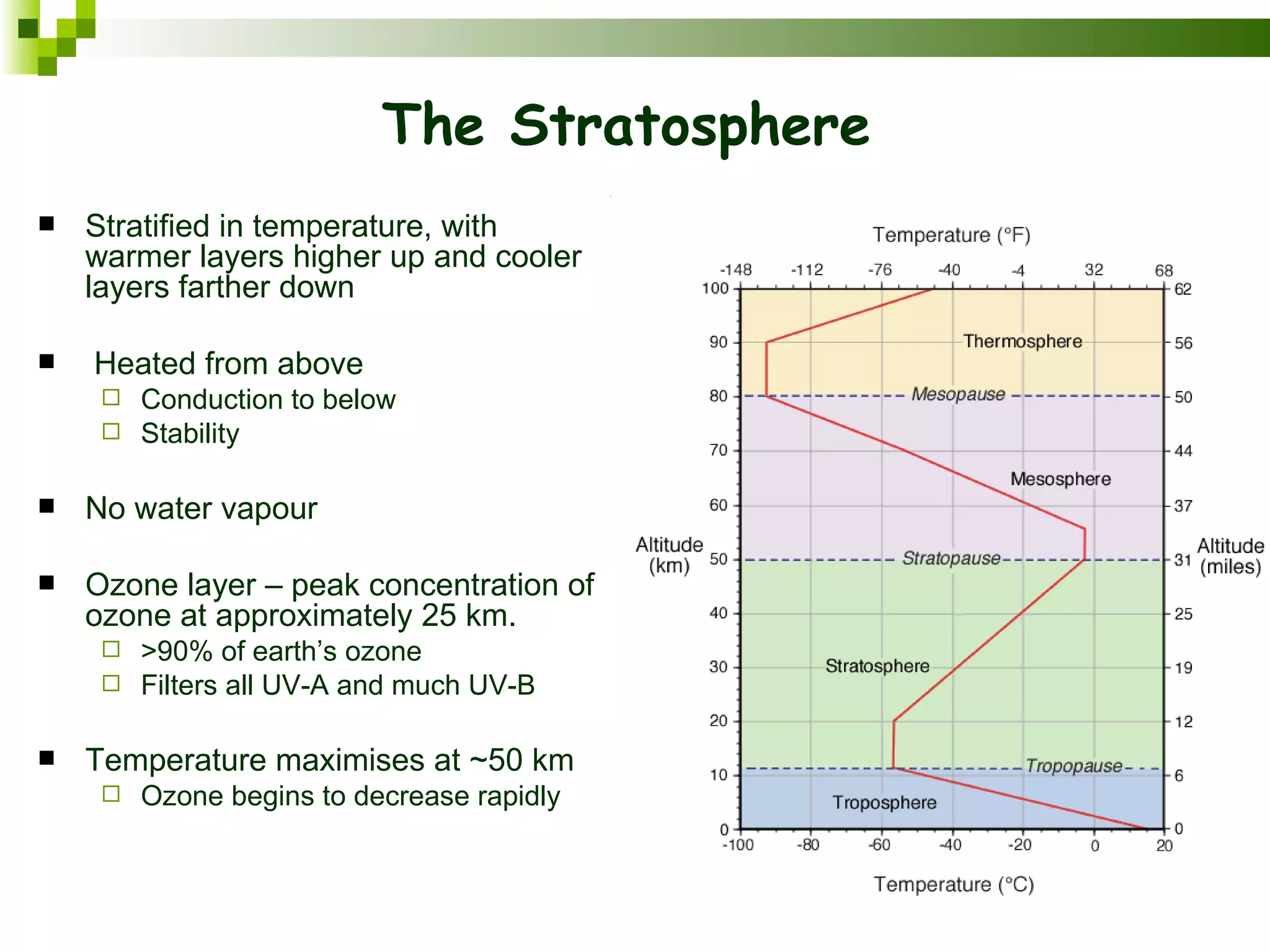
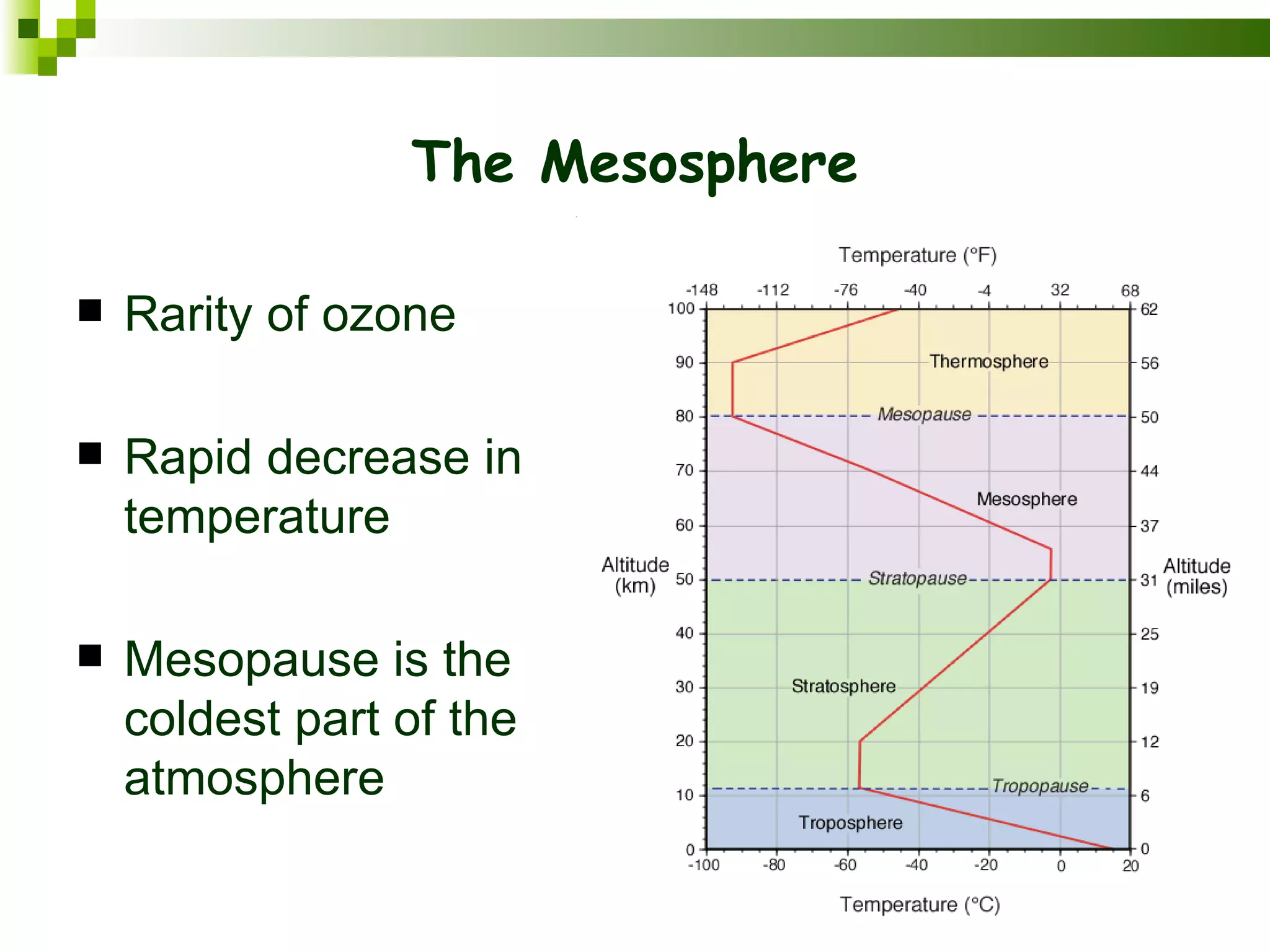
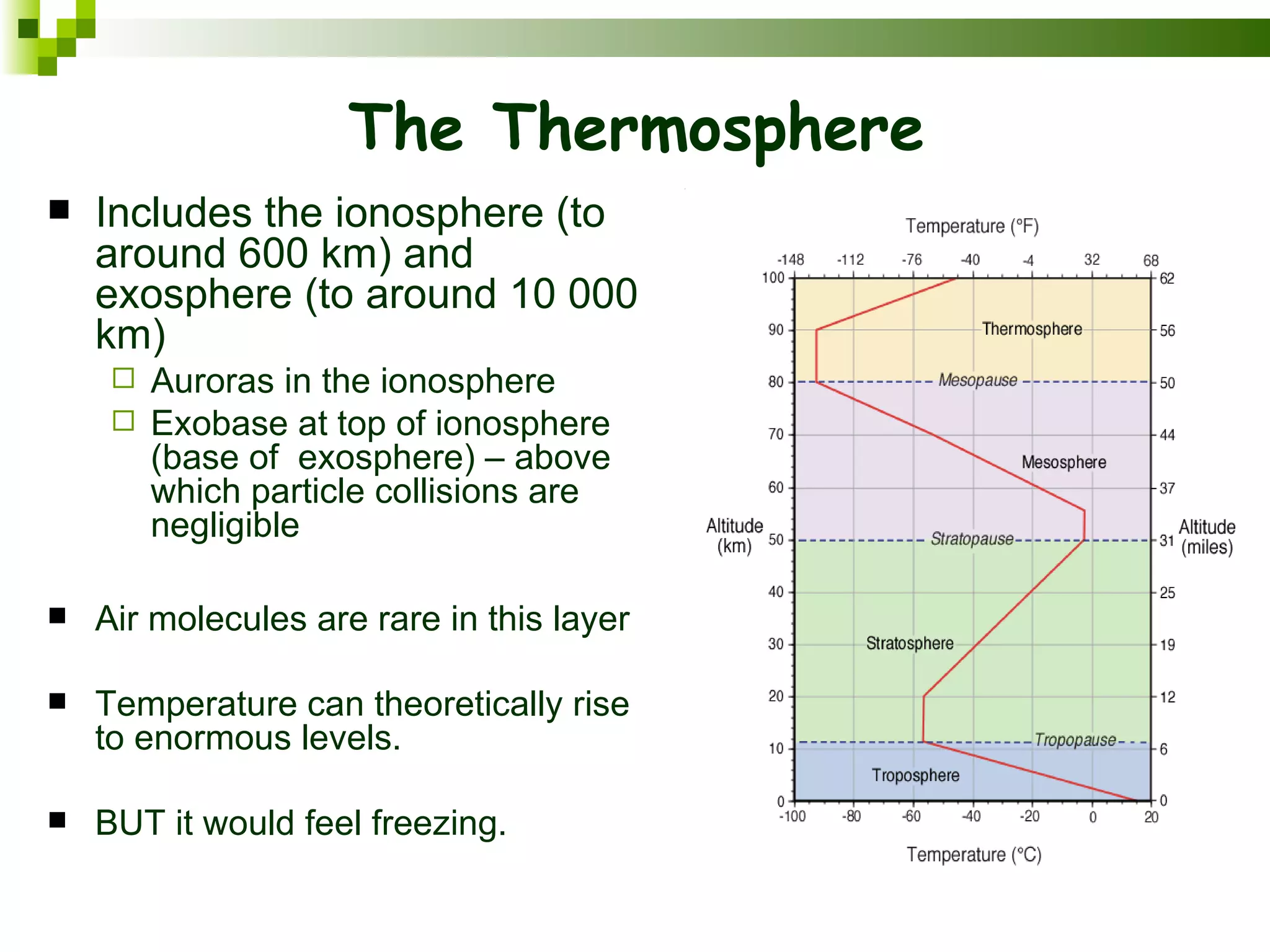
The document summarizes the composition and structure of the atmosphere. It discusses that the atmosphere is composed of 78% nitrogen, 21% oxygen and trace amounts of other gases. It also notes there are variable gases like water vapor and particulates suspended in the air. The structure of the atmosphere consists of four layers - the troposphere closest to the surface which contains most of the atmosphere, the stratosphere above it which contains the ozone layer, the mesosphere and thermosphere above that. Each layer has distinctive temperature characteristics.