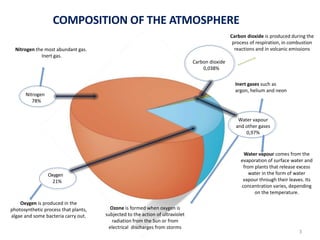
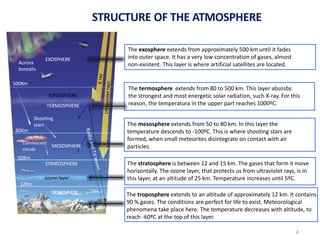
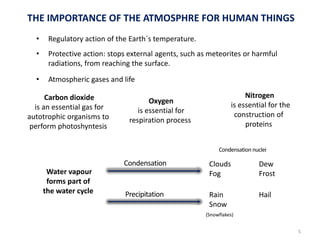
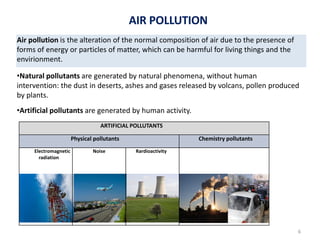
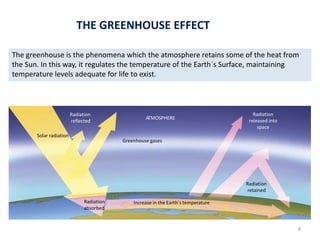
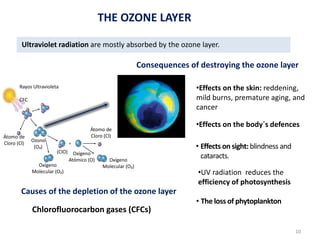
The atmosphere is the gaseous layer surrounding the Earth. It is composed primarily of nitrogen (78%) and oxygen (21%), along with smaller amounts of other gases like carbon dioxide and water vapor. The atmosphere protects life on Earth by regulating temperature, blocking harmful radiation, and enabling processes like photosynthesis. Air pollution from human activities like burning fossil fuels threatens ecosystems and human health through effects like acid rain and respiratory problems. The greenhouse effect retains some of the Sun's heat in the lower atmosphere, maintaining temperatures suitable for life; however, increased greenhouse gases are causing global warming and its consequences.